Session Information
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: Methotrexate (MTX) is currently the recommended first-line conventional DMARD for the treatment of JIA with oligo- or polyarthritis. There are limited data on how or when to stop MTX monotherapy for well-controlled JIA. The aims of this study were to 1) describe the MTX withdrawal strategies used for well-controlled JIA in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) registry, 2) compare time to flare among different MTX withdrawal strategies, and 3) evaluate the duration of disease remission after MTX withdrawal.
Methods: The study population consisted of patients enrolled in the CARRA Registry with a diagnosis of non-systemic JIA on MTX monotherapy for at least 12 months and with 2 consecutive registry visits with no reported disease activity (based on joint count = 0 and no changes in medication). Subjects with systemic JIA, non-JIA rheumatic diseases or inflammatory bowel disease were excluded. Subjects were divided into 3 cohorts: 1) MTX continuation, 2) MTX tapering, or 3) MTX discontinuation without taper. Rates of flare (new or increased JIA medication, > 0 joint count) were compared among groups using Kaplan-Meier curves and multivariable Cox regression, adjusted for demographic, disease, and medication history covariates. Rates of remission were also compared between subjects who stopped MTX without taper and subjects who tapered MTX, using descriptive statistics.
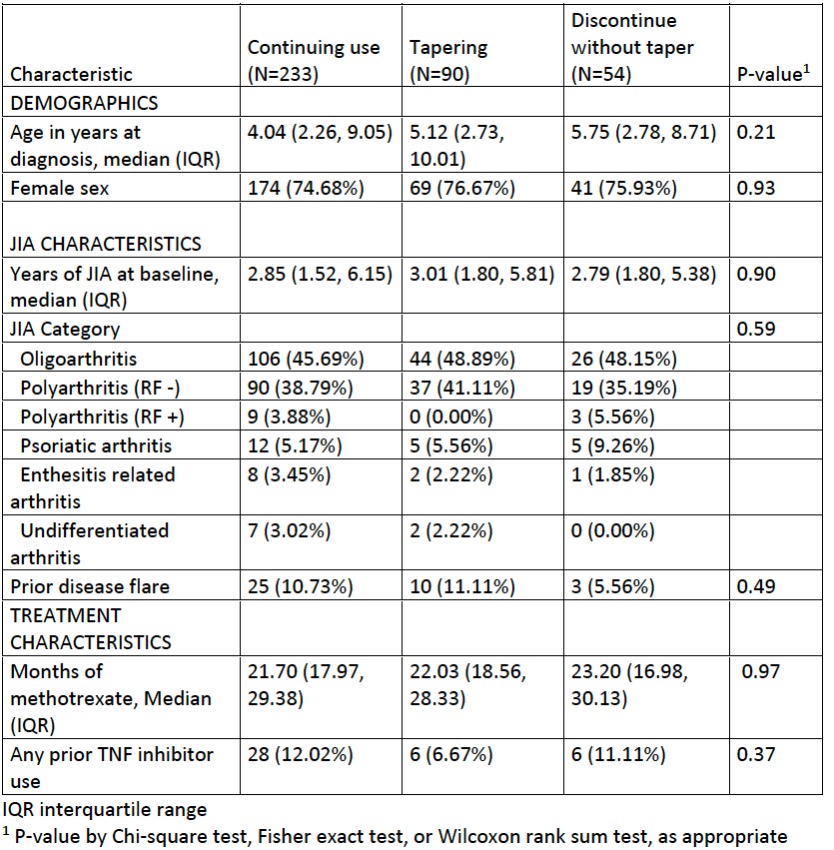
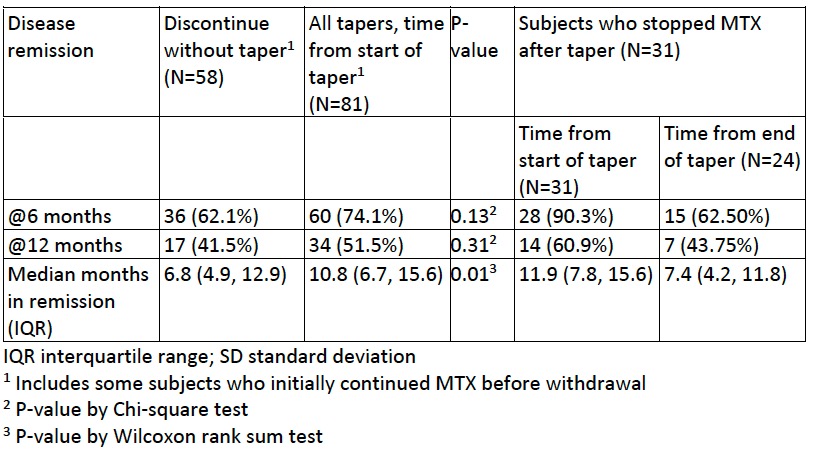
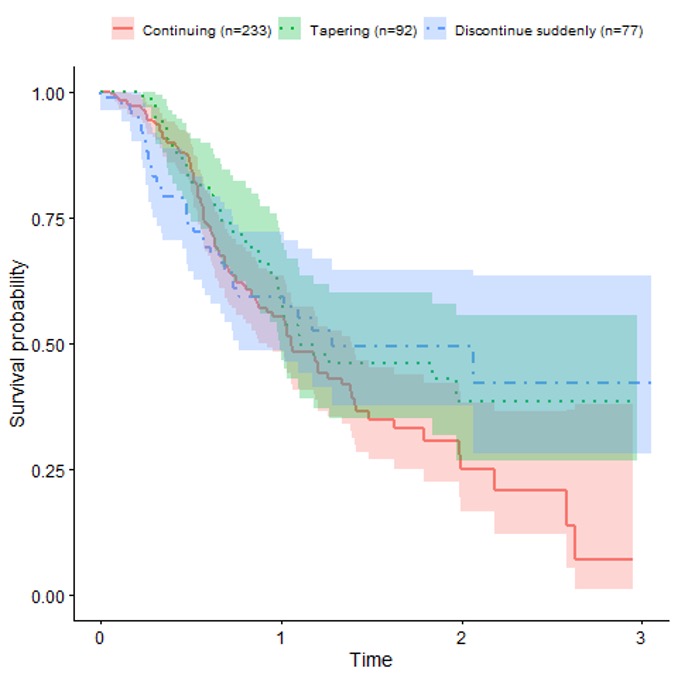
Results: Of 9835 persons in the CARRA Registry, 377 fulfilled the study criteria. MTX monotherapy was continued in 233 (61.8%), tapered in 90 (23.9%) and stopped in 54 (14.3%). Of MTX continuers, 25 (10.7%) were subsequently observed to withdraw MTX (2 tapered, 23 stopped). Subject characteristics (Table 1) did not differ significantly among the groups. Most subjects (85%) had oligoarthritis or RF – polyarthritis, consistent with overall JIA enrollment distribution. Prior TNF inhibitor use was less common among those who tapered, while prior flares were less commonly recorded among those who stopped MTX without taper. No association was found between flare of JIA and MTX withdrawal strategy in unadjusted analyses (Figure) or adjusted models (taper vs. continuation: 0.89, 95% CI 0.59-1.34; discontinuation vs. continuation: 0.90, 95% CI 0.54-1.48). Rates of sustained remission and median time in remission were modestly higher among those who tapered MTX compared to those who stopped MTX (Table 2). Among those who were in sustained remission after tapering MTX, rates of remission were higher before tapering than afterwards (Table 2).
Conclusion: Within the CARRA Registry, we did not observe significant differences in time to flare between subjects with non-systemic JIA who continued MTX and those who tapered or stopped MTX. This may reflect bias from residual confounding. Rates of sustained remission were modestly higher during times of MTX tapering compared to times after discontinuation.
To cite this abstract in AMA style:
Twilt M, Baszis K, Ringold S, Beukelman T, Dennos A, Shrader P, Horton D. Methotrexate Withdrawal and Outcomes in Participants with Well-controlled Non-systemic JIA Within the CARRA Registry [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/methotrexate-withdrawal-and-outcomes-in-participants-with-well-controlled-non-systemic-jia-within-the-carra-registry/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/methotrexate-withdrawal-and-outcomes-in-participants-with-well-controlled-non-systemic-jia-within-the-carra-registry/