Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Methotrexate (MTX) is frequently prescribed with biologic disease-modifying antirheumatic drugs. Evidence has shown that tocilizumab (TCZ) monotherapy is effective in the treatment of patients with rheumatoid arthritis (RA).1,2 Similar outcomes have been shown in patients responding to TCZ combination therapy who discontinued MTX or remained on combination therapy.3 The purpose of this study was to examine MTX discontinuation and dose decreases in patients with RA initiating TCZ and to describe disease activity outcomes in a real-world setting.
Methods: TCZ-naïve patients enrolled in the Corrona RA Registry who initiated TCZ and had a 6-month follow-up visit without discontinuation of TCZ were included. Patients were grouped by MTX dose at the time of TCZ initiation (≤ 10 mg, > 10 to ≤ 15 mg, > 15 to ≤ 20 mg, > 20 mg). The primary outcome was the proportion of patients with changes in MTX use at 6 months. Changes in disease activity (Clinical Disease Activity Index [CDAI]) and patient-reported outcomes (PROs) over the follow-up period are described.
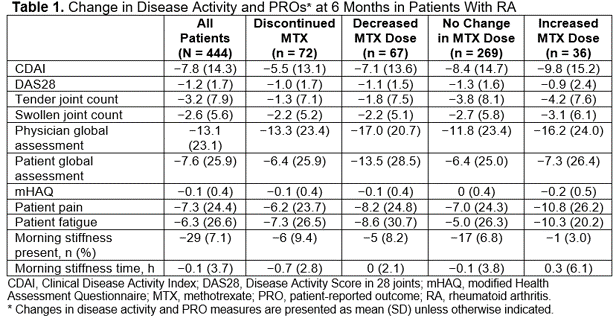
Results: Of 444 eligible patients, 82.7% were female, and 83.7% were white, with a mean (SD) disease duration of 11.6 (9.3) years and a baseline CDAI score of 24.0 (15.4). The mean (SD) MTX dose at baseline was 17.7 (5.8) mg. Overall, a total of 139 patients (31.3%) discontinued or decreased MTX at 6 months (overall mean [SD] dose change, −3.0 [7.5] mg); across baseline MTX dose groups, the proportion of patients who discontinued or decreased MTX at 6 months ranged from 28.2% to 38.2%. Improvements in CDAI scores and PROs were observed at 6 months in all baseline MTX dose groups and in patients who discontinued, decreased, maintained, or increased MTX doses at 6 months (Table 1). Similar patterns and results were observed at 12 months (not shown).
Conclusion: A considerable proportion of patients initiating TCZ were able to discontinue or decrease the dose of MTX after TCZ initiation. Patients who were able to discontinue or decrease MTX experienced similar improvements in disease activity and functionality. Discontinuing or decreasing MTX may be an effective treatment strategy for patients initiating TCZ combination therapy.
References:
- Bykerk VP, et al. Clin Rheumatol. 2015;34(3):563-571.
- Jones G, et al. Ann Rheum Dis. 2010;69(1):88-96.
- Kremer J, et al. Arthritis Rheumatol. 2018;70(8):1200-1208.
Acknowledgments: Support for third-party writing assistance, furnished by Health Interactions, Inc, was provided by Genentech, Inc.
To cite this abstract in AMA style:
Pappas D, Blachley T, Zlotnick S, Best J, Emeanuru K, Kremer J. Methotrexate Discontinuation and Dose Decreases After Therapy with Tocilizumab: Results from the Corrona Rheumatoid Arthritis Registry [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/methotrexate-discontinuation-and-dose-decreases-after-therapy-with-tocilizumab-results-from-the-corrona-rheumatoid-arthritis-registry/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/methotrexate-discontinuation-and-dose-decreases-after-therapy-with-tocilizumab-results-from-the-corrona-rheumatoid-arthritis-registry/