Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: The extent to which adherence to prescribed treatment regimens contributes to differential disease outcomes in JIA – and demographic disparities in these outcomes – is unknown, largely because adherence is challenging to assess. Many methods rely on patient or caregiver report, which tend to overestimate adherence. Pharmacy dispensing data offer an objective means of assessing adherence through metrics such as the medication possession ratio (MPR), which measures the proportion of time a patient has medication available. We aimed to link medication adherence estimates to clinical data in the electronic health record (EHR) to investigate associations between adherence, patient characteristics, and disease activity.
Methods: This single-center retrospective cohort study leveraged pharmacy dispensing data from the EHR, provided by Surescripts, to calculate MPRs for MTX for patients with a physician diagnosis of JIA in a large pediatric rheumatology clinic in the US. Surescripts is an information technology company that supports electronic prescriptions and provides these data to subscribing institutions.
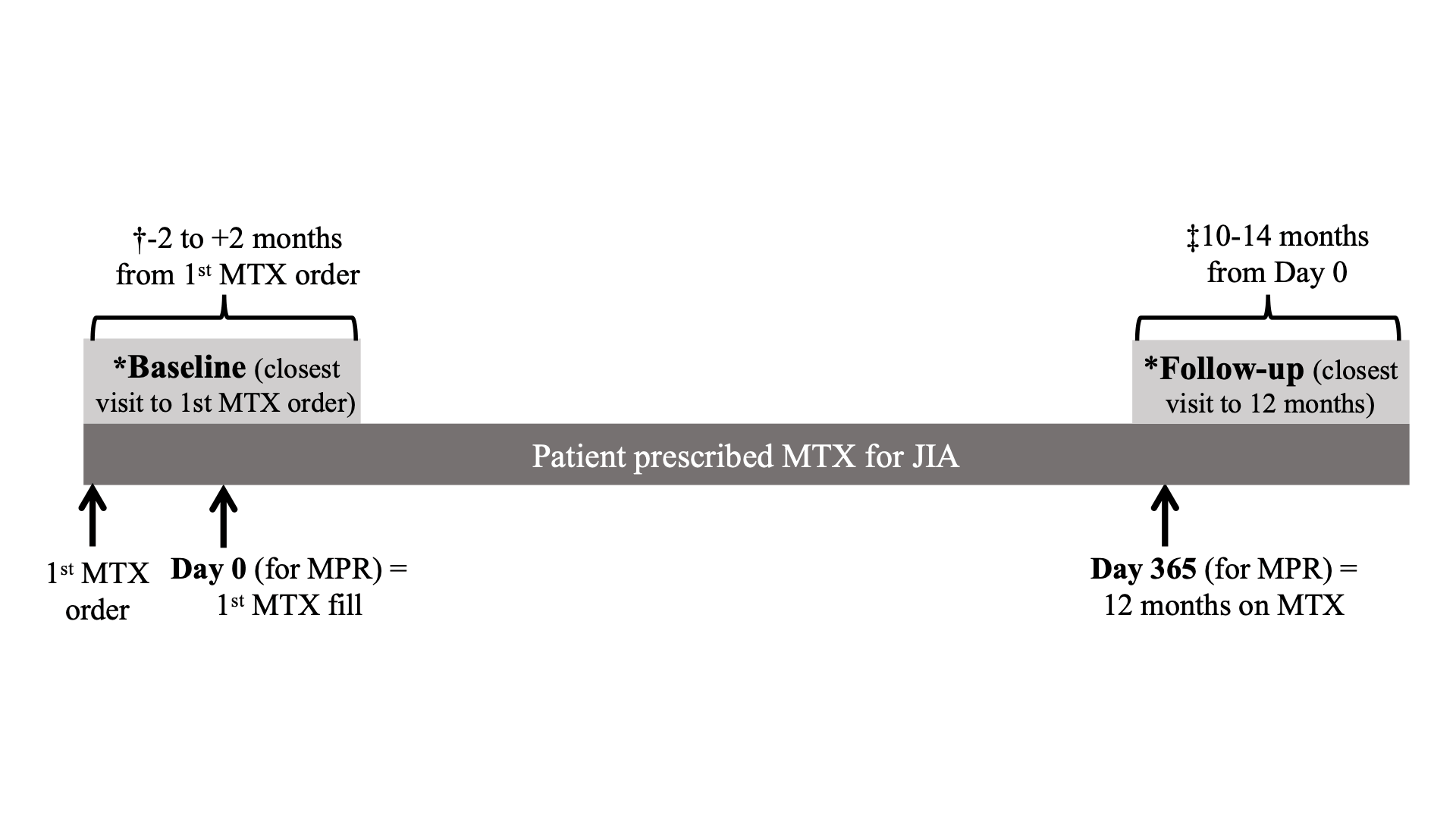
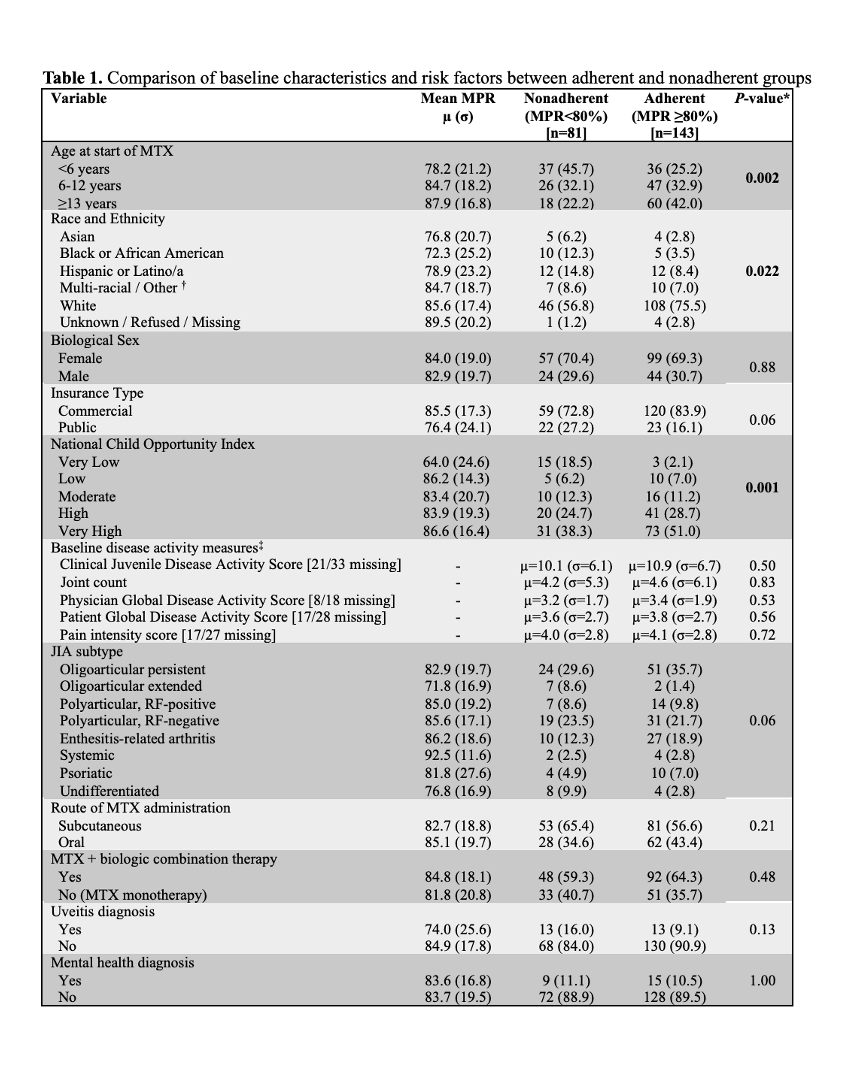
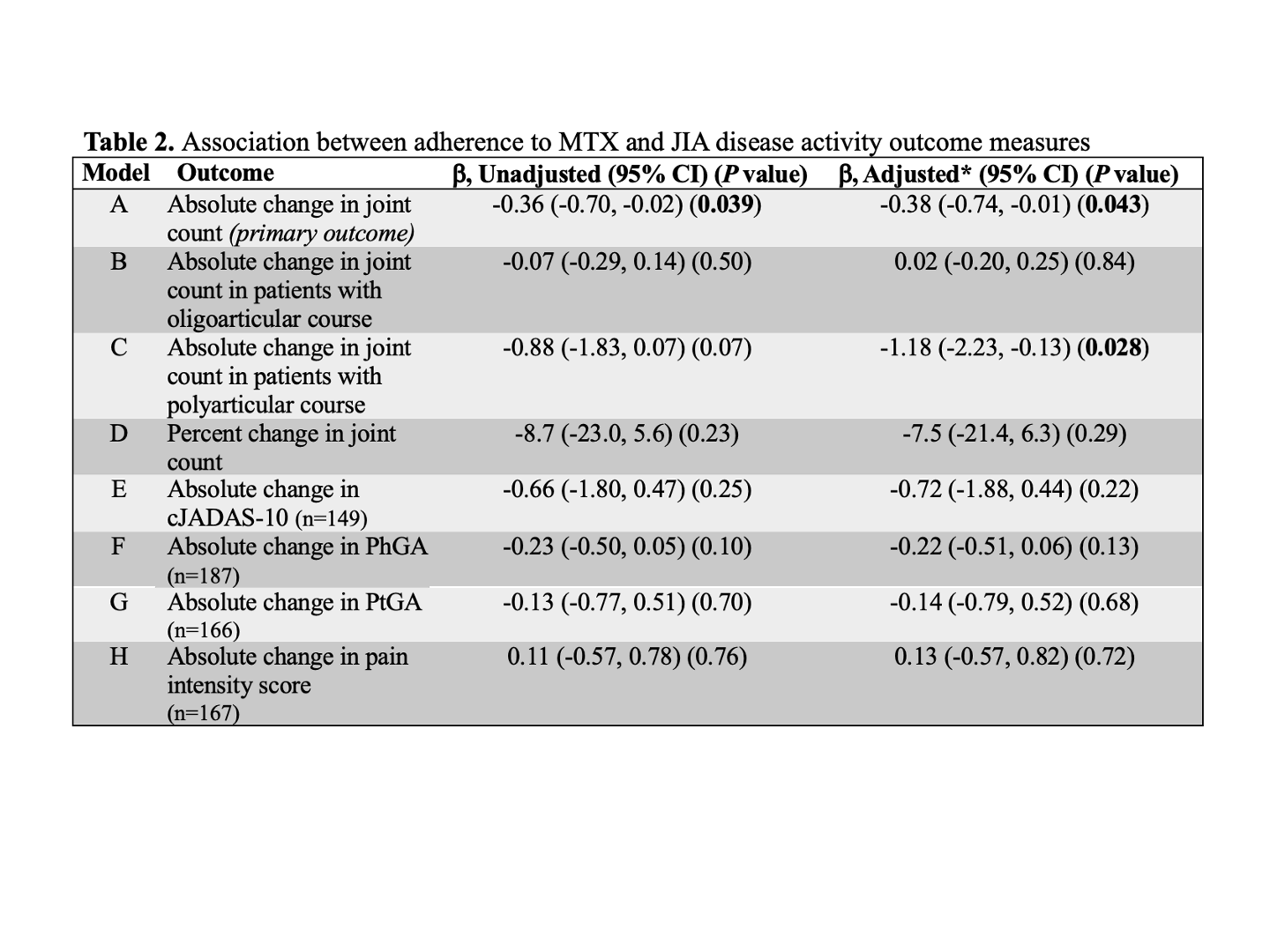
All incident users of MTX, treated between 1/2016 and 9/2023 for ≥12 months with outpatient rheumatology visits within 2 months of the first MTX prescription and after 12 months of follow up (±2 months) (Figure 1), were included in the sample. MPR was calculated using the total days’ supply over a fixed 365-day interval following the initial MTX prescription fill date. Patient-level variables (Table 1), including sociodemographic and visit-level clinical factors from the baseline and follow up visits, were extracted from the EHR. We used Fisher’s exact and Wilcoxon rank-sum tests to compare patient characteristics between adherent (MPR ≥80%) and nonadherent (MPR < 80%) groups and multivariable linear regression to investigate associations between MPR and disease activity outcomes measures. Our primary outcome was change in joint count from baseline to 12-month follow up.
Results: Among 224 patients included in the analysis, the mean MPR was 83.7%. 81 patients (36.2%) were classified as nonadherent. In bivariate analysis (Table 1), patients of younger age, Black race, and from areas with lower child opportunity index (COI) were more likely to be classified as nonadherent. Unadjusted mean change in joint count was -4.0 joints. In multivariable analysis (Table 2), active joint count decreased by 0.38 more joints in the adherent compared to nonadherent group (95% CI -0.74, -0.01) and by 1.18 more joints in the adherent group among patients with polyarticular course (95% CI -2.23, -0.13).
Conclusion: Linking dispense data to clinical EHR data offers an accessible, objective method for evaluating adherence to chronic medications. We identified demographic and area-level determinants of adherence, along with small but statistically significant differences in JIA disease activity measures by adherence status. Future work is needed to evaluate adherence as a potential mediator of known outcome disparities for socially disadvantaged populations. Our qualitative work exploring patient perspectives on mechanisms that influence medication adherence will inform future adherence-focused interventions.
*Visits from which disease activity data were obtained
†Patients without a visit between _2 and +2 months from the first MTX order were excluded
‡Patients without a visit between the 10- and 14-month window were excluded
95% CI, 95% confidence interval; cJADAS_10, clinical Juvenile Arthritis Disease Activity Score 10; PhGA, physician global assessment of disease activity score; PtGA, patient or parent global assessment of overall well-being score.
To cite this abstract in AMA style:
Abel D, Anderson D, Kallan M, Utidjian L, Burnham J, Chang J, Kenyon C, Gmuca S. Methotrexate Adherence in JIA: Use of Electronic Health Record-Linked Pharmacy DispensingData [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/methotrexate-adherence-in-jia-use-of-electronic-health-record-linked-pharmacy-dispensingdata/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/methotrexate-adherence-in-jia-use-of-electronic-health-record-linked-pharmacy-dispensingdata/