Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: As female patients constitute a significant proportion of rheumatoid arthritis (RA) cases, pregnancy management is crucial for women with RA. This study aimed to investigate medication use patterns according to pregnancy outcomes and identify factors associated with abnormal pregnancy outcomes (APO) in female RA patients in Korea.
Methods: Using the Korean national health insurance database, female RA patients aged between 20 and 50 were identified based on the presence of both RA diagnostic codes and prescriptions for any disease-modifying anti-rheumatic drug (DMARD). Pregnancy episodes were selected using diagnostic or procedure codes and divided into two groups: the delivery group and the APO group (including abortion and stillbirth). Characteristics were compared between the two groups, and medication use patterns were analyzed during the preconception period, pregnancy, and one year after delivery. A multivariable logistic regression analysis was conducted to identify factors associated with APO.
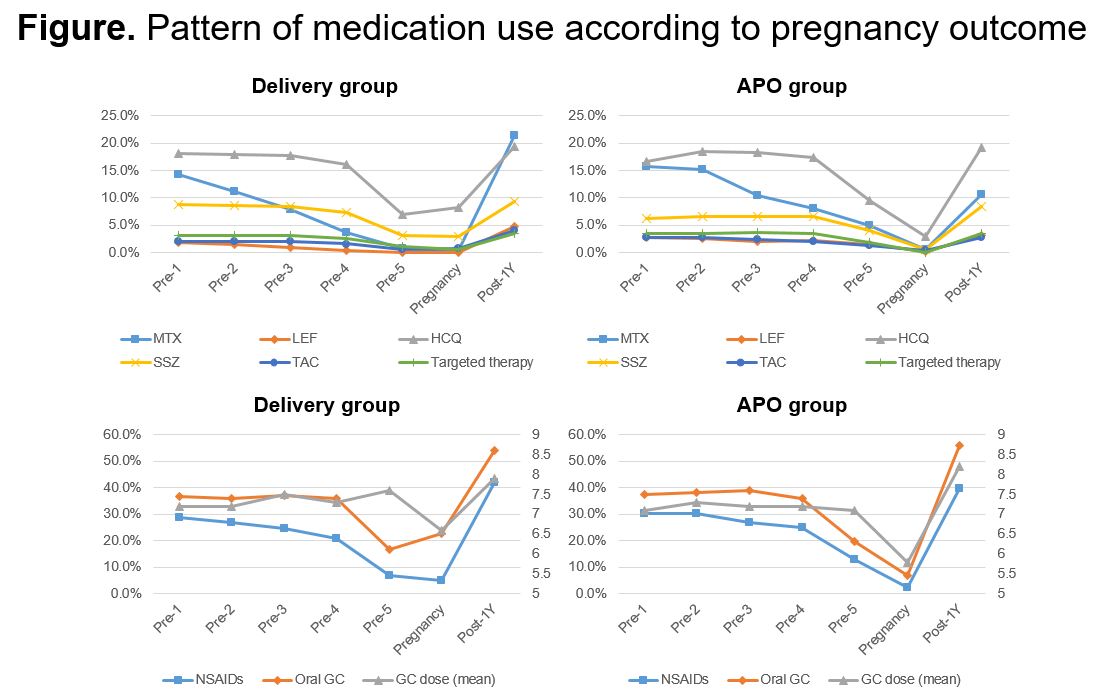
Results: A total of 5,728 pregnancy episodes were included, with 4,576 in the delivery group and 1,152 in the APO group. The mean maternal age for all pregnancy episodes was 33.7 years, which was higher in the APO group. Comorbidities such as chronic pulmonary disease and diabetes mellitus were more frequent in the APO group. Hydroxychloroquine was the most commonly used conventional synthetic DMARD during the preconception period and pregnancy in both groups. Except for sulfasalazine and targeted therapies, all DMARDs were more frequently used in the APO group during the preconception period. Methotrexate and leflunomide prescriptions during pregnancy were nearly nonexistent in the delivery group. Within one year after delivery or termination of pregnancy, there was a rapid increase in the use of all DMARDs and oral glucocorticoids. In the multivariable analysis, patients aged 30-39 (adjusted odds ratio [aOR] 1.36, 95% confidence interval [CI] 1.09-1.68) and 40-49 (aOR 5.41, 95% CI 4.21-6.95) had a higher risk of APO compared to those aged 20-29. Methotrexate use (aOR 2.19, 95% CI 1.60-2.99) and leflunomide use (aOR 2.87, 95% CI 1.49-5.54) within three months before conception were associated with APO. Hospital visits for RA during pregnancy were associated with a lower risk of APO (aOR 0.67, 95% CI 0.55-0.82).
Conclusion: Medication use patterns differed between the delivery and APO groups. Methotrexate and leflunomide were associated with a higher risk of APO, emphasizing the importance of appropriate medication adjustment when planning for pregnancy. Regular hospital visits for RA treatment during pregnancy are also crucial in reducing the risk of APO.
APO, abnormal pregnancy outcome; MTX, methotrexate; LEF, leflunomide; HCQ, hydroxychloroquine; SSZ, sulfasalazine; TAC, tacrolimus; NSAID, nonsteroidal anti-inflammatory drug; GC, glucocorticoid.
(Pre_1: from 12 months to 9 months before conception, Pre_2: from 9 months to 6 months before conception, Pre_3: from 6 months to 3 months before conception, Pre_4: during 3 months before conception, Pre-5: from conception to pregnancy recognization, Pregnancy: during pregnancy, Post_1Y: during 1 year after pregnancy outcome)
To cite this abstract in AMA style:
Song Y, Cho S, Jung Y, Keum J, Jung S, Yoo D, Sung Y. Medication Use Patterns and Factors Associated with Abnormal Pregnancy Outcomes in Patients with Rheumatoid Arthritis in Korea [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/medication-use-patterns-and-factors-associated-with-abnormal-pregnancy-outcomes-in-patients-with-rheumatoid-arthritis-in-korea/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/medication-use-patterns-and-factors-associated-with-abnormal-pregnancy-outcomes-in-patients-with-rheumatoid-arthritis-in-korea/