Session Information
Session Type: Abstract Session
Session Time: 10:00AM-10:50AM
Background/Purpose: RAPID3 (routine assessment of patient index data) on an MDHAQ (multiple multidimensional health assessment questionnaire) was developed initially to assess patients with rheumatoid arthritis (RA) according to agreement with the DAS28 (disease activity score 28).1 Routine completion of self-report MDHAQ/RAPID3 by patients with all diagnoses prior to seeing the physician was found to be the most effective strategy for MDHAQ completion by all RA patients in routine care.2 This led to recognition that RAPID3 was clinically informative to describe clinical status and change in patients with all rheumatic diagnoses,3 such as evidence of a similar disease burden in osteoarthritis (OA) and RA patients.4 Another index of MDHAQ scores, FAST3 (fibromyalgia assessment screening tool 3) has been developed to screen for fibromyalgia (FM) on the same MDHAQ that includes RAPID3, with agreement of about 80% with formal 2011 revised FM criteria.5 We examined RAPID3 levels in patients seen in routine care at one academic site, including all patients and those with OA, RA, systemic lupus erythematosus (SLE), and other diagnoses, in subsets of patients who were FM positive or negative
Methods: All patients with all diagnoses seen at one academic rheumatology site complete an MDHAQ at all visits. The MDHAQ includes 0-10 scores for physical function (FN), pain (PN), patient global assessment (PATGL), fatigue (FT), 0-54 self-report rheumatoid arthritis disease activity index (RADAI) painful joint count, and 0-60 symptom checklist (SX). MDHAQ/RAPID3 (0-30) =FN+PN+PATGL; RAPID3 severity categories include high >12, moderate=6.1-12, low=3.1-6 and remission≤3. MDHAQ/FAST3-P is a 0-3 cumulative index to screen for FM: 1 point each for PN≥6, RADAI≥16, and SX≥16 (2/3=FM). The 2011 FM criteria are based on 2 questionnaires, somatic symptom scale and widespread pain index, compiled into a polysymptomatic distress scale. RAPID3 scores were classified by severity categories in all patients and in subsets with OA, RA, SLE or other diagnoses, according to positive or negative 2011 FM criteria or MDHAQ FAST3-P FM criteria. Statistical analyses: Chi-square test for comparisons of percentages and Kruskal-Wallis test for RAPID3 distributions between diagnoses
Results: Median RAPID3 was 12.5 (high severity) in all patients, but only 9.3 (moderate severity) in 423 patients (74.3%) who did not meet 2011 FM criteria and 8.7 (moderate severity) in 406 patients (71.4%) who did not meet FAST3-P FM criteria (Table). By contrast, median RAPID3 was 19.0 (high severity) in 146 patients (25.7%) who met 2011 FM criteria and 19.8 in 163 patients (28.9%) who met FAST3-P FM criteria (Table). Similar patterns were seen in patients with OA, RA or SLE or other diagnoses (Table)
Conclusion: Patients who have comorbid FM have substantially elevated scores for RAPID3. Results of treatment in rheumatology clinical trials and clinical care may be underestimated by inclusion of patients with FM; routine FAST3-P screening for FM might be desirable.
References
1.J Rheumatol. 2006;33(11):2146-52
2.Clin Exp Rheumatol. 2007;25(6 Suppl 47):69-81
3.Rheum Dis Clin North Am. 2009;35(4):829-42, x-xi
4.RMD Open. 2017;3:e000391
5.ACR Open Rheumatol. 2019;1(8):516-25
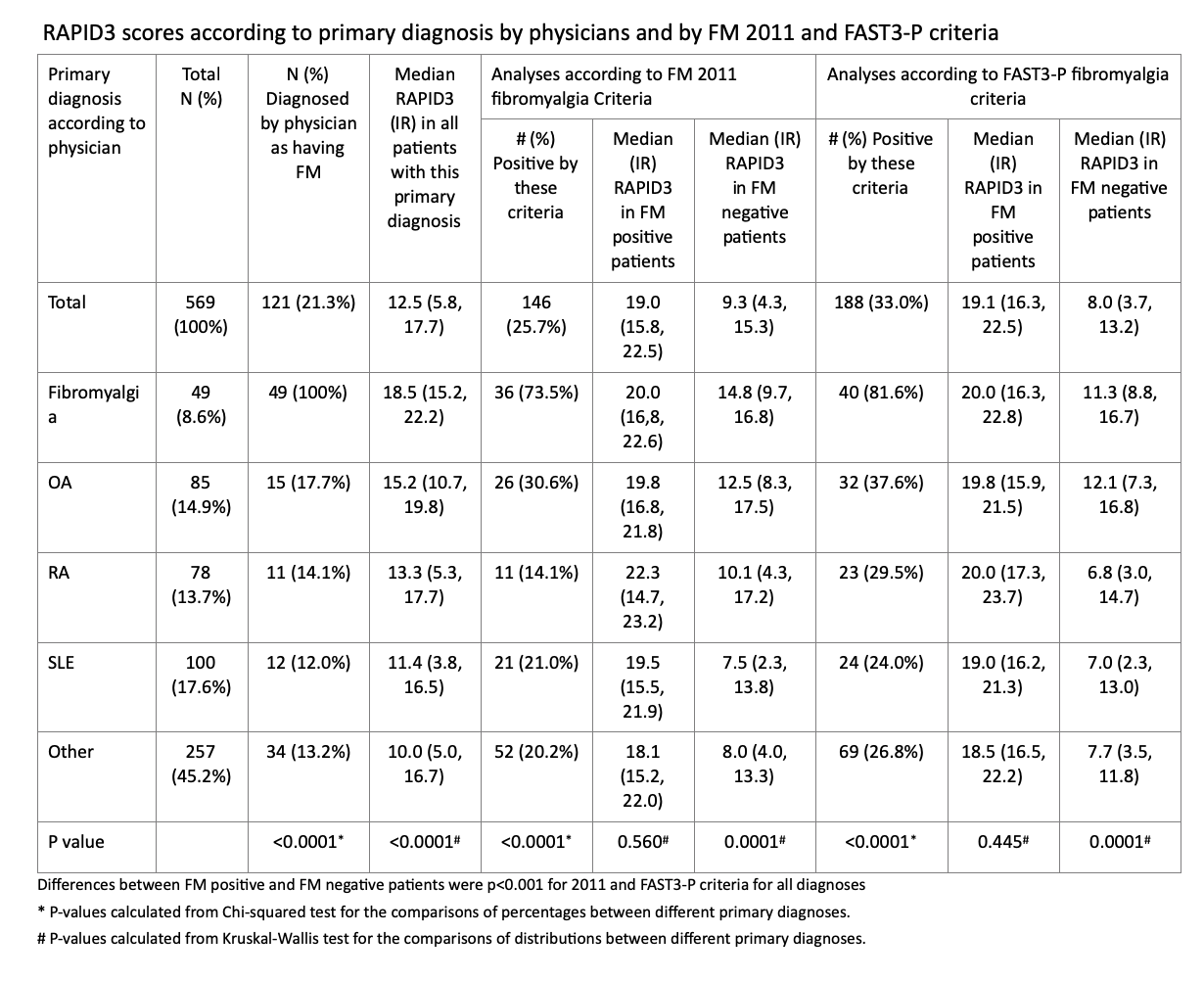 Table 1. RAPID3 scores according to primary diagnosis by physicians and by FM 2011 and FAST3-P criteria
Table 1. RAPID3 scores according to primary diagnosis by physicians and by FM 2011 and FAST3-P criteria
To cite this abstract in AMA style:
Schmukler J, Li T, Schroeder K, Pincus T. MDHAQ/RAPID3 (multidimensional Health Assessment Questionnaire/routine Assessment of Patient Index Data) Levels Are Elevated in Rheumatology Patients Who Meet 2011 Fibromyalgia (FM) or MDHAQ/FAST3 (fibromyalgia Assessment Screening Tool) Criteria [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/mdhaq-rapid3-multidimensional-health-assessment-questionnaire-routine-assessment-of-patient-index-data-levels-are-elevated-in-rheumatology-patients-who-meet-2011-fibromyalgia-fm-or-mdhaq-fast3-fi/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/mdhaq-rapid3-multidimensional-health-assessment-questionnaire-routine-assessment-of-patient-index-data-levels-are-elevated-in-rheumatology-patients-who-meet-2011-fibromyalgia-fm-or-mdhaq-fast3-fi/
