Session Information
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: Fatigue is a major symptom for rheumatoid arthritis (RA) patients with a high prevalence (40%-70%)1 measured by different validated questionnaires and scales. A 0-10 visual analog scale (0-10) was reported as a fine tool, with a cut-off >5/10 indicating high fatigue2.A residual fatigue has been described in a significant proportion of patients in low activity/remission of the disease by different indices3.
We studied the association between high level of residual fatigue (VAS>5/10) and disease variables including self-reported burden of disease and residual fatigue with different indices in a group of non – selected RA patients.
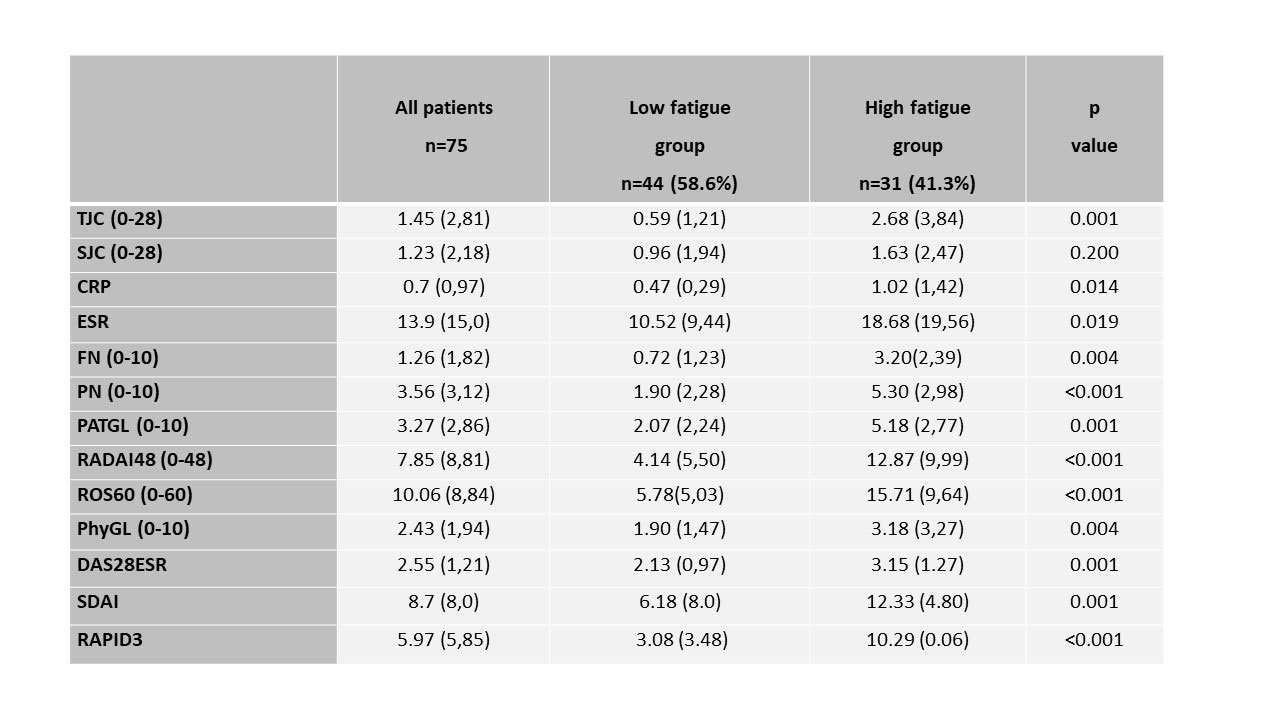
Methods: RA patients (ACR/EULAR criteria, 2010) were included consecutively in an Arthritis Unit during a period of 3 months. Patients completed a 2-page MDHAQ (multidimensional health assessment questionnaire) that includes a 0-10 fatigue VAS; cut point of >5/10 was regarded as high fatigue. Other queries were: physical function (FN) + pain (PN) + patient global assessment (PATGL), checklist of 60 symptoms (ROS60) and self-assessment 48 joint count (RADAI48). Demographic variables (age, sex, BMI) and clinical variables: disease duration, treatment with bDMARDs and glucocorticoids, tender joint counts (TJC 0-28), swollen joint count (SJC 0-28), acute phase reactants (ESR, CRP), physician global assessment (PhyGL) were also collected. Clinical activity indices (DAS28ESR, SDAI and RAPID3) and their respective clinically significant differences between groups (DAS28ESR >0.6; SDAI >10; RAPID3 >3.8) were calculated.
Descriptive statistics (mean and SD) and bivariate analyses (Student’s t-test for quantitative and Chi2 tests for categorical variables) were analyzed to compare groups with fatigue >or< than 5, of numerical differences between indices and the frequency of high fatigue in patients with low disease activity for the 3 studied indices.
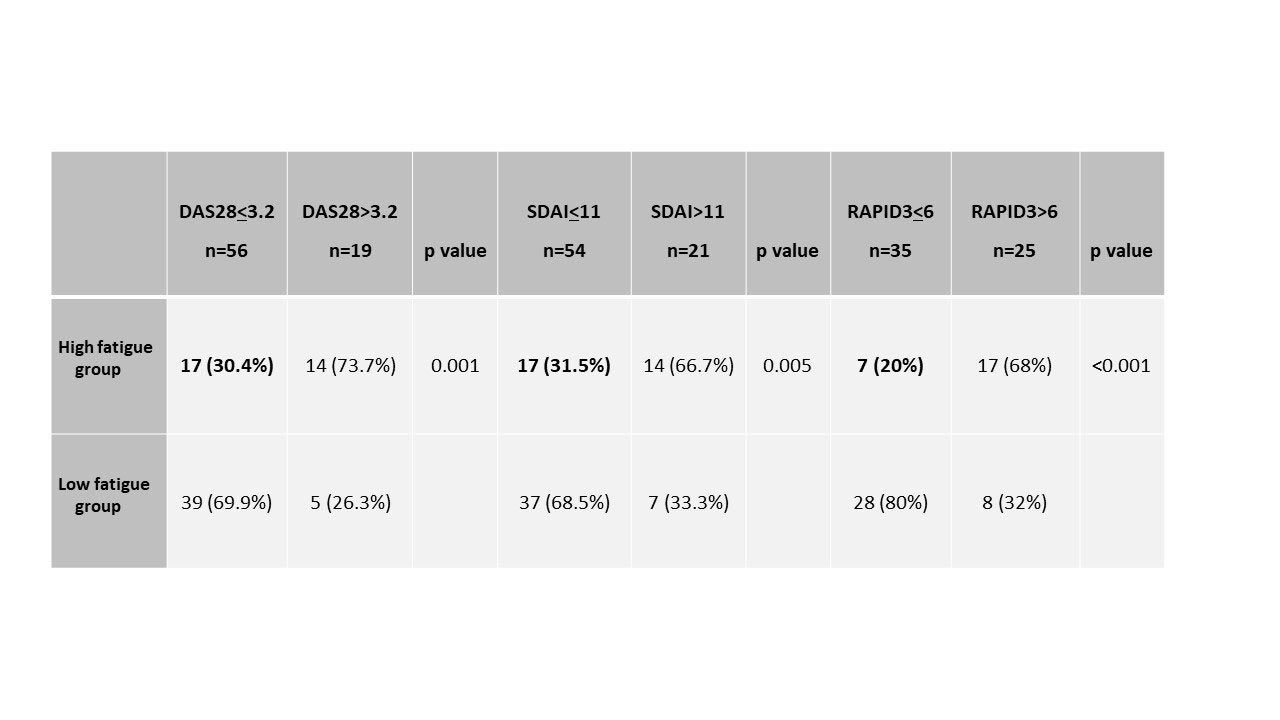
Results: A total of 75 RA patients (84% females) were studied; mean age 62 (SD:11.6) years, median BMI 22.8 (SD:8.0), mean disease duration 4.6 (SD:5) years, 64% with bDMARD and 43.5% with glucocorticoids treatment. Global mean of clinical indices were: DAS28ESR=2.55 (SD:1.21), SDAI=8.7 (SD:8.00) and RAPID3=5.97 (SD:5.85). High fatigue (VAS-fatigue > 5/10) was seen in 31(41.2%) of the patients associated with poorer disease activity variables including self-reported burden of disease than low fatigue patients, except for SJC (table 1). Mean differences in the activity indices studied between the high and low fatigue groups were statistically significant and clinically relevant for DAS28ESR=1.02 ( >0.6) and RAPID3=7.2 ( >3.8), but not for SDAI=6.15 ( < 10). The mean disease activity were moderate by SDAI=12.33 (SD:4.80) and RAPID3=10.29 (SD:0.06) and remission by DAS28ESR=3.15 (SD:1.27) in the high-fatigue group and the percentage of low activity/remission were 30.4% by DAS28ESR, 31.5% by SDAI and only 20% by RAPID3 (table 2).
Conclusion: High fatigue in RA patients was associated with a worse self-reported disease burden. We highlight the use of RAPID3 as a stricter activity index than others to capture residual fatigue.
References:1.Hewlett S. Rheumatology 2011. 2.Pollard LC. Rheumatology 2006. 3.Druce K. Rheumatology2016.
To cite this abstract in AMA style:
Morlà R, Frade-Sosa B, Sapena N, Gomez-Puerta J, Sanmarti R, Pincus T. MDHAQ/RAPID3 Is a Feasible Tool to Estimate High Comorbid Fatigue and Residual Fatigue in Rheumatoid Arthritis Patients [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/mdhaq-rapid3-is-a-feasible-tool-to-estimate-high-comorbid-fatigue-and-residual-fatigue-in-rheumatoid-arthritis-patients/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/mdhaq-rapid3-is-a-feasible-tool-to-estimate-high-comorbid-fatigue-and-residual-fatigue-in-rheumatoid-arthritis-patients/