Session Information
Date: Monday, November 13, 2023
Title: Abstracts: Pediatric Rheumatology – Clinical II: Connective Tissue Disease
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Craniofacial scleroderma is a rare subtype of linear scleroderma affecting the head and neck. Up to 40% of patients experience extracutaneous manifestations (ECMs), but radiographic characterization of non-CNS involvement is not well described. Existing clinical assessment rubrics prioritize cutaneous changes, likely underestimating the extent of disease-related manifestations. We sought to categorize and quantify the incidence of non-CNS ECMs detected on maxillofacial MRI in a prospective cohort of patients with craniofacial scleroderma. We hypothesize that maxillofacial MRI would identify facial involvement beyond standard clinical examination.
Methods: We performed retrospective review of 120 patients with Craniofacial Scleroderma enrolled in the prospective National Registry of Childhood Onset Scleroderma (IRB #19080297). Inclusion criteria included baseline maxillofacial MRI with concurrent clinical 3D image.
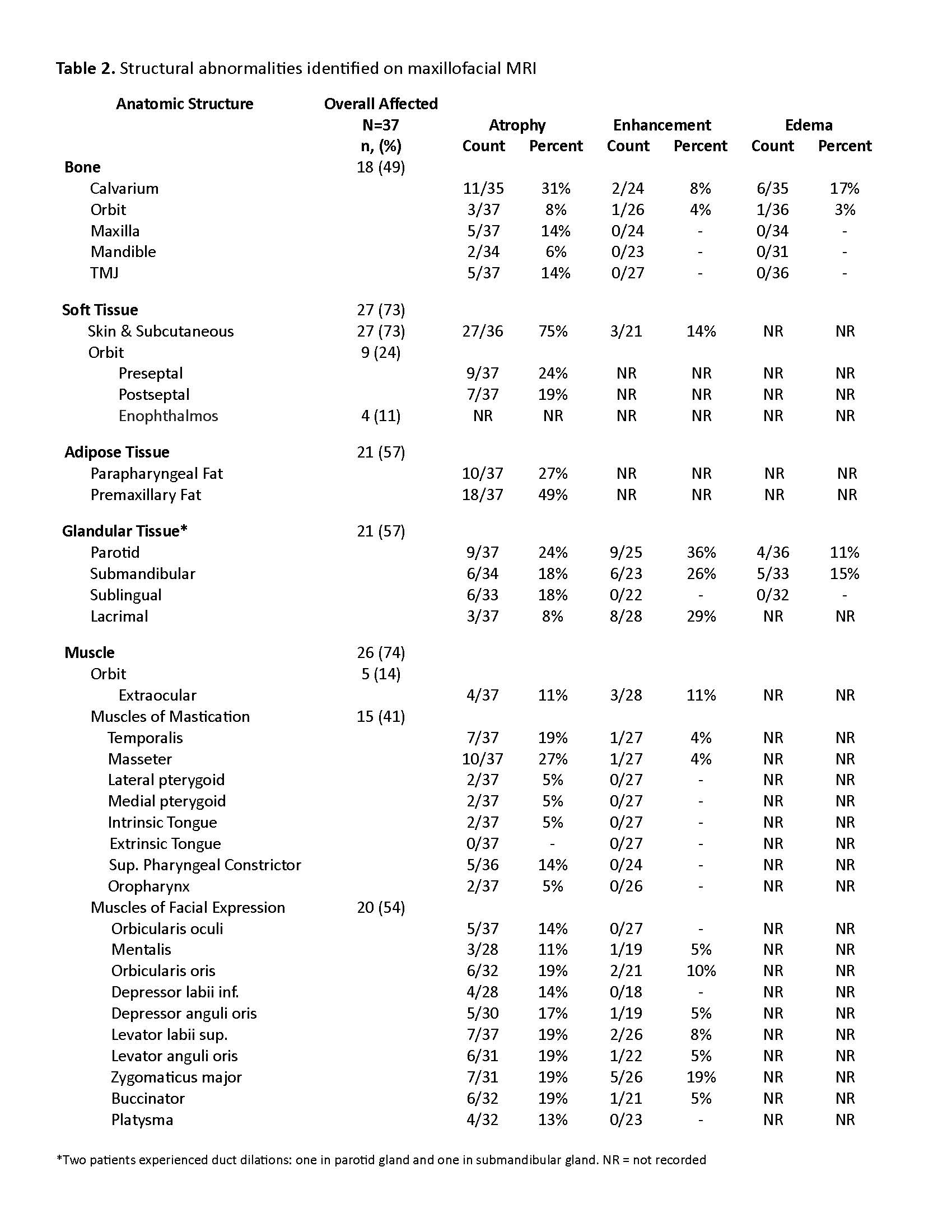
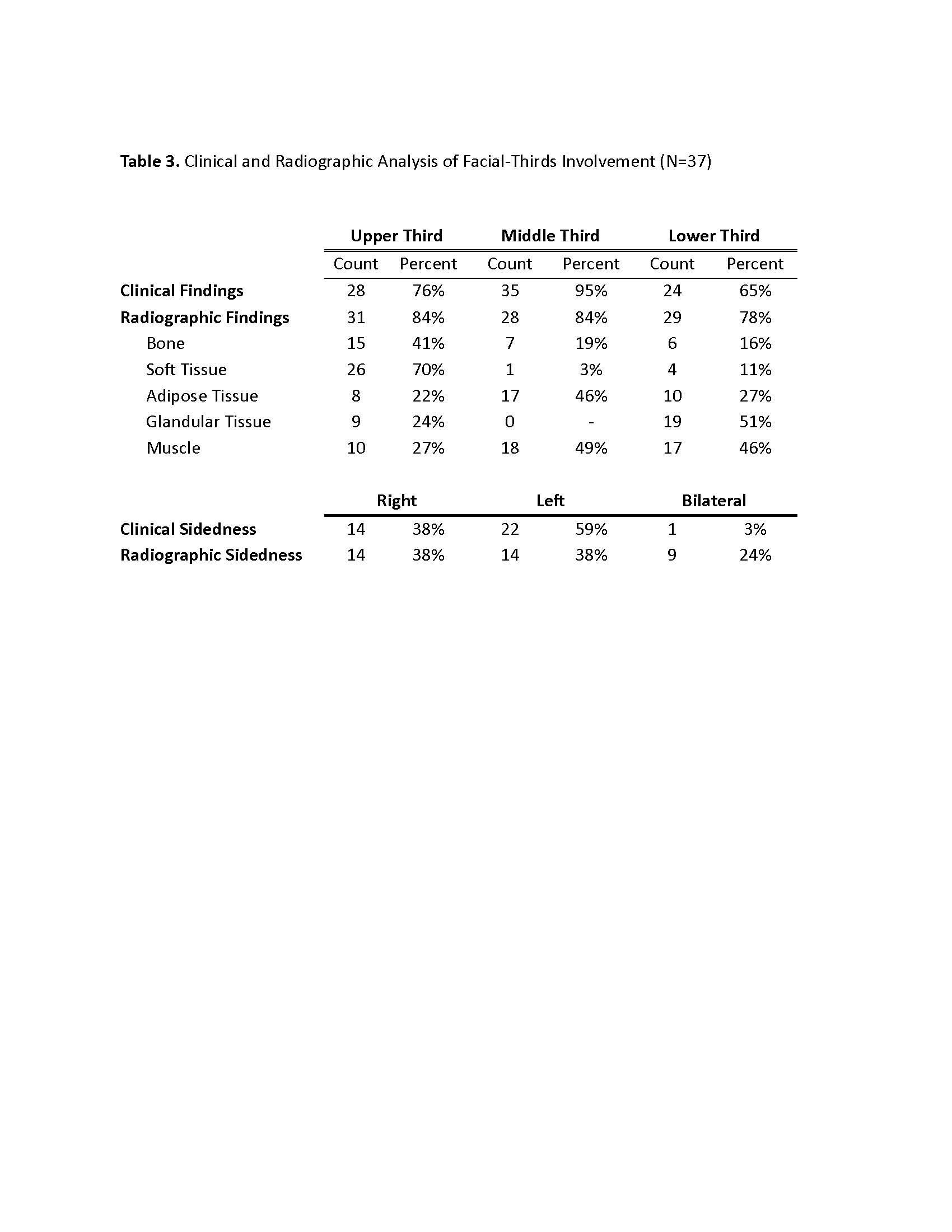
Laterality of visible facial involvement (left, right, midline) and superior to inferior facial lesion distribution (top, middle, bottom thirds) were recorded from review of 3-dimensional digital facial images. Top third was defined as the area between horizontal lines drawn through trichion and glabella, middle third between glabella and subnasale, and bottom third between subnasale and mention. Evaluation of bone, soft tissue, fat, glands, and muscles were categorized as atrophy, edema, or abnormal enhancement.
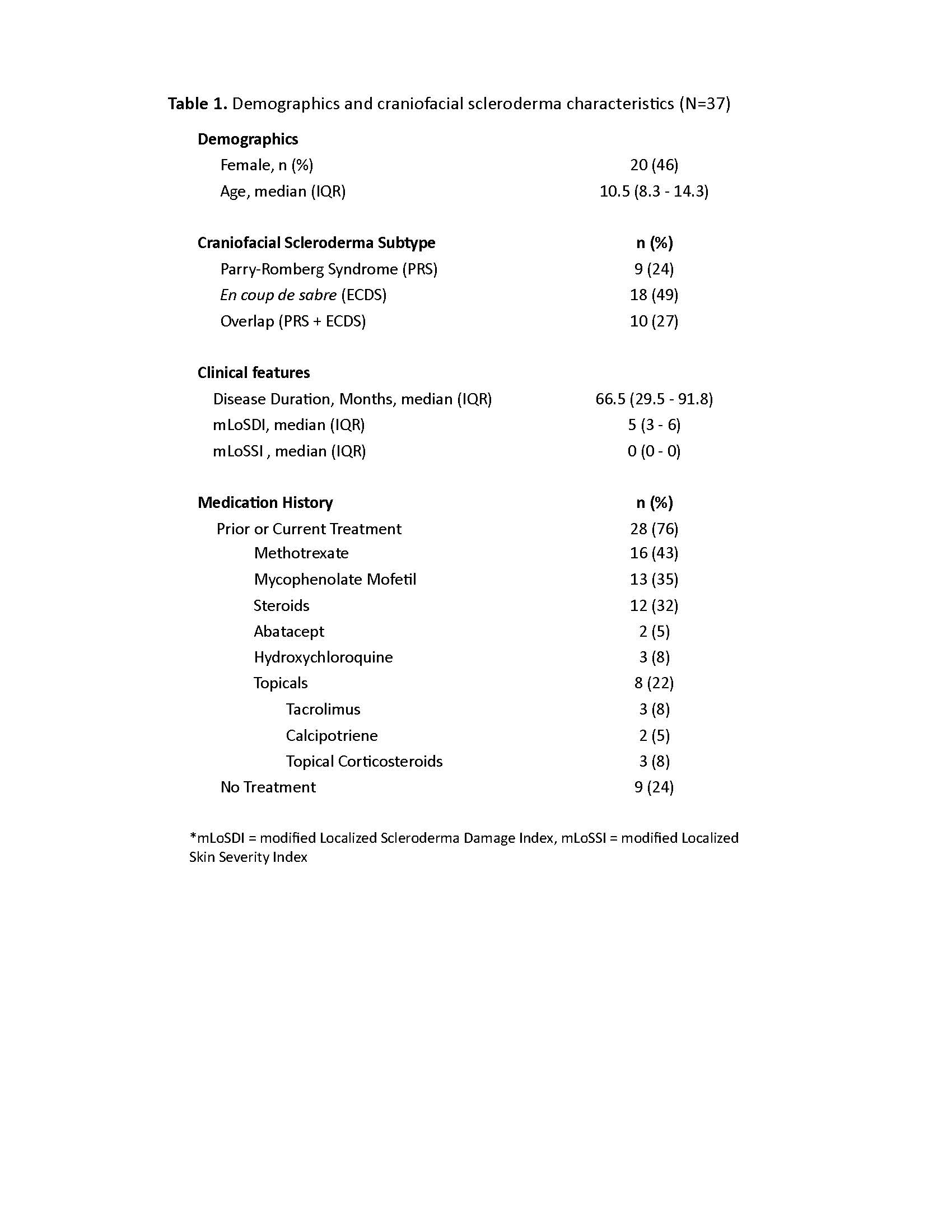
Results: Thirty-seven patients met imaging criteria for inclusion, (age range 4-20 years, median age 10.5 years) (Table 1). Overall, structural abnormalities were frequently detected on maxillofacial MRI (bone=49%, soft tissue=73%, adipose=57%, gland=57% and muscle=74%) (Table 2). Atrophy was the most common finding, particularly in the skin/subcutis (75%) and adipose tissues (57%). Enhancement was frequently observed in glandular tissues (parotid 36%, lacrimal 29%) as well as in the muscles of facial expression. MRI demonstrated increased frequency of bilateral involvement compared to clinical assessment (24% vs 3%). Radiographic changes were more frequently seen compared to clinical abnormalities in the upper-third (84% vs 76%) and lower-third (78% vs 65%) (Table 3). Frequencies of radiographic abnormalities differed across facial thirds, with soft tissue changes most frequent in the upper third (70%), muscle changes in the middle third (49%), and glandular changes in the lower third (51%).
Conclusion: Our study demonstrated an overall high incidence of facial bone, soft tissue, glandular, and muscle involvement in craniofacial scleroderma. Clinical examination and radiographic review frequently demonstrated discordant results. Therefore, we recommended baseline maxillofacial MRI in addition to brain MRI to better delineate the full extent of disease involvement in craniofacial scleroderma patients. Future work should assess evolution of these findings over time, response to immunomodulatory treatment, and development of multi-modal assessment rubrics.
To cite this abstract in AMA style:
Glaser D, Metry A, DeRosas E, Subramanian S, Torok K. Maxillofacial MRI Augments Detection of Non-CNS Abnormalities in Pediatric Craniofacial Scleroderma [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/maxillofacial-mri-augments-detection-of-non-cns-abnormalities-in-pediatric-craniofacial-scleroderma/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/maxillofacial-mri-augments-detection-of-non-cns-abnormalities-in-pediatric-craniofacial-scleroderma/