Session Information
Session Type: Late-Breaking Abstract Session
Session Time: 11:30AM-1:00PM
Background/Purpose: T helper (Th)1 and Th17 lymphocytes play a role in the pathogenesis of giant cell arteritis (GCA). Current treatments (e.g., corticosteroids and tocilizumab) target primarily the Th17 axis, leaving substantial residual Th1 activity. Preclinical research in human GCA models implicate granulocyte macrophage colony stimulating factor (GM-CSF), an upstream mediator of both Th1 and Th17 cells, as a pathogenic factor for GCA. We evaluated the efficacy and safety of mavrilimumab (anti GM-CSF receptor α monoclonal antibody) for maintaining disease remission in patients with GCA.
Methods: Patients with active new onset (N/O) or relapsing refractory (R/R) GCA were enrolled in a randomized, double-blind, placebo-controlled phase 2 trial. GCA was confirmed by temporal artery biopsy or vascular imaging. Active disease was defined as presence of GCA symptoms and erythrocyte sedimentation rate (ESR) (≥30 mm/hr) and/or C-reactive protein (CRP) (≥1 mg/dL) elevation within 6 weeks from randomization. Corticosteroid-induced remission (resolution of GCA symptoms and CRP < 1 mg/dL or ESR < 20 mm/hr) was required by baseline. Patients were randomized 3:2 to receive mavrilimumab 150 mg or placebo subcutaneously every two weeks in addition to a protocol-defined 26-week prednisone taper starting at 20-60 mg/day. Stratification by disease type (N/O vs. R/R) was applied.
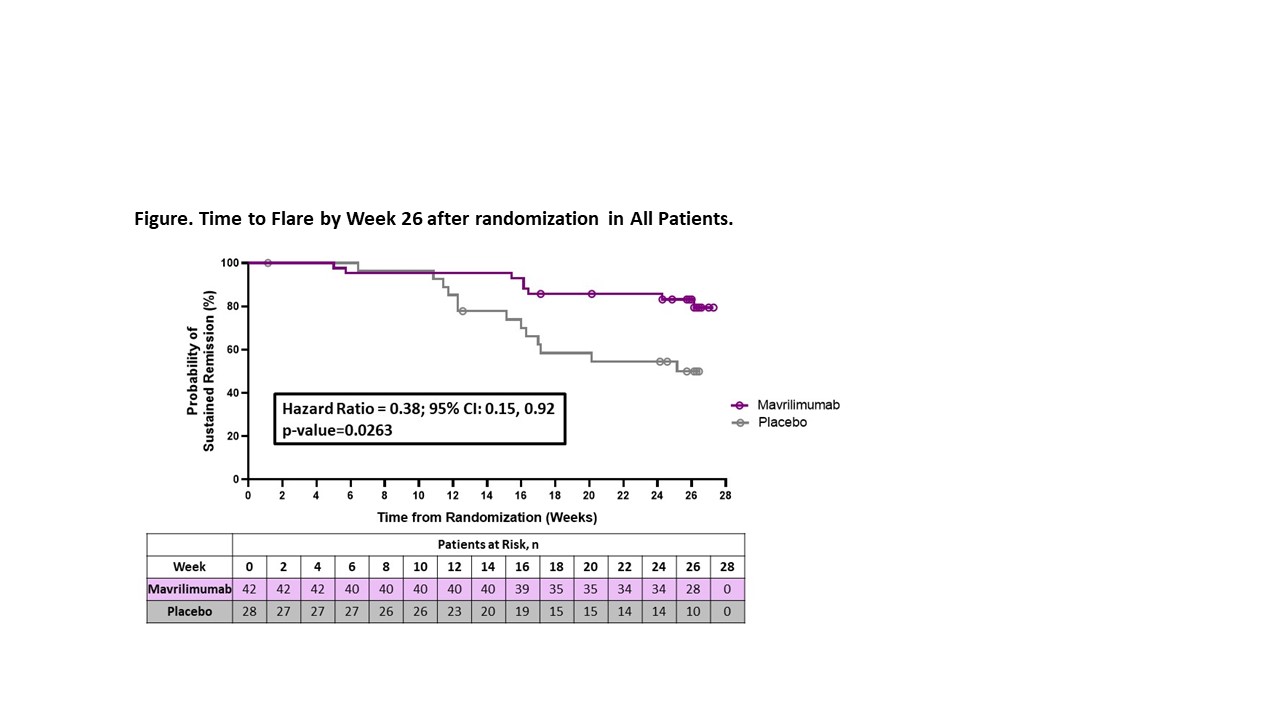
The primary efficacy endpoint was time to first flare by Week 26 in all treated patients (modified intention-to-treat population). Flare, confirmed by an independent adjudication committee, was defined as ESR ≥30 mm/hr and/or CRP ≥1 mg/dL and re-appearance of GCA symptoms or new/worsening vasculitis determined by imaging. Sustained remission through Week 26 was a key secondary endpoint. Safety up to Week 30 was assessed.
Results: 70 patients (35 N/O, 35 R/R) were randomized to mavrilimumab (N=42) or placebo (N=28). Mean (SD) age was 69.7 (7.48) years and 71.4% were female. Disease flare by Week 26 occurred in 8 (19%) and 13 (46.4%) patients receiving mavrilimumab and placebo, respectively (27.4% reduction). Median time to flare by Week 26 could not be estimated in the mavrilimumab group due to too few events (Not Estimable [NE]) and was 25.1 weeks [95% CI: (16.0, NE)] in the placebo group (HR [95% CI] 0.38 [0.15, 0.92]; p=0.0263) (Figure). Sustained remission at Week 26 occurred in 83.2% of the patients receiving mavrilimumab and in 49.9% of those receiving placebo (33.4% increase; p=0.0038). Results were consistent across disease type subgroups (HR for flare: N/O patients 0.29 [95% CI: 0.06, 1.31; nominal p= 0.0873]; R/R patients 0.43 [95% CI: 0.14, 1.30]; nominal p=0.1231) (Table 1). Adverse events (AEs), mostly mild to moderate in severity, were comparable between groups. There were 5 serious AEs (mavrilimumab 2 [4.8%], placebo 3 [10.7%]), none drug-related (Table 2). No deaths or vision loss occurred.
Conclusion: Mavrilimumab was superior to placebo on the primary and secondary efficacy endpoints of time to flare and sustained remission at Week 26 in patients with GCA. Mavrilimumab was well tolerated, and no new safety signals were observed. Results with this novel treatment are encouraging for the management of GCA.
To cite this abstract in AMA style:
Cid M, Unizony S, Pupim L, Fang F, Pirrello J, Ren A, Samant M, Zhou T, Paolini J. Mavrilimumab (anti GM-CSF Receptor α Monoclonal Antibody) Reduces Time to Flare and Increases Sustained Remission in a Phase 2 Trial of Patients with Giant Cell Arteritis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/mavrilimumab-anti-gm-csf-receptor-%ce%b1-monoclonal-antibody-reduces-time-to-flare-and-increases-sustained-remission-in-a-phase-2-trial-of-patients-with-giant-cell-arteritis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/mavrilimumab-anti-gm-csf-receptor-%ce%b1-monoclonal-antibody-reduces-time-to-flare-and-increases-sustained-remission-in-a-phase-2-trial-of-patients-with-giant-cell-arteritis/