Session Information
Date: Monday, November 9, 2020
Title: Miscellaneous Rheumatic & Inflammatory Diseases Poster III: Therapies
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: Infusion reactions and other adverse events associated with pegloticase may lead to discontinuation of treatment in patient populations that have already failed or are intolerant to other uric acid lowering therapies (ULTs). Maximizing the benefit of pegloticase is critical in the absence of other suitable ULTs. Here, we examine use of pegloticase for patients in US clinical care and identify variables associated with longer time on therapy.
Methods: The ARN-TRIO Rheumatology registry contains EMR (fielded and open text), lab, procedure, infusion, medical claims, and specialty pharmacy data generated in care of >75,000 patients by ARN, a network of independent practices with >200 rheumatologists across the US. This study included data for gout-diagnosed patients who initiated their last pegloticase course between Jul 2015 and Oct 2019 with >180 days follow-up from pegloticase initiation (index). A course was defined as pegloticase infusions spaced < 90 days apart. Chart reviews were conducted for all study patients to determine treatment status (ongoing vs. discontinued) and reasons for discontinuation. To assess consistency of control of sUA < 6 mg/dL, evaluations were limited to patients with 2+ sUA measures during treatment. Time to event analyses were by Kaplan-Meier and log-rank or Wilcoxon test.
Results: 110 of 213 pegloticase-treated patients met study criteria. [TABLE 1] At time of assessment, 84% (95) had discontinued treatment; 19% (18/95) discontinued as intended upon meeting treatment goals, 39% (37/95) lack or loss of efficacy, 16% (15/95) adverse events, 8% (8/95) lost to follow up, 2% (2/95) each for cost or payer denial and unrelated health issues, and 17% (16/95) unknown. Of the 15 patients who discontinued therapy due to adverse events, 80% (12) cited infusion and/or allergic reactions. Median times to discontinuation were 20 weeks overall and 22 weeks for non-planned discontinuation. Controlled sUA (< 2 sUA ≥6 mg/dL) and concurrent use of immune-modulating therapies (predominantly methotrexate) were associated with a significantly longer pegloticase duration. [FIGURES 1 & 2] Variables NOT associated with unplanned discontinuation were race, gender, age, payer type, kidney disease, osteoarthritis or osteoporosis, baseline sUA, and pegloticase infusion schedule. None of the 12 patients who discontinued due to infusion or allergic reactions received concurrent immune-modulating therapies.
Conclusion: These observations suggest that treatment with pegloticase may be lengthened with concomitant use of immune-modulating therapies; however, a larger scale prospective study should be done to further elucidate the benefits of immunomodulation beyond durability of treatment, including safety benefits such as minimal to no infusion reactions.
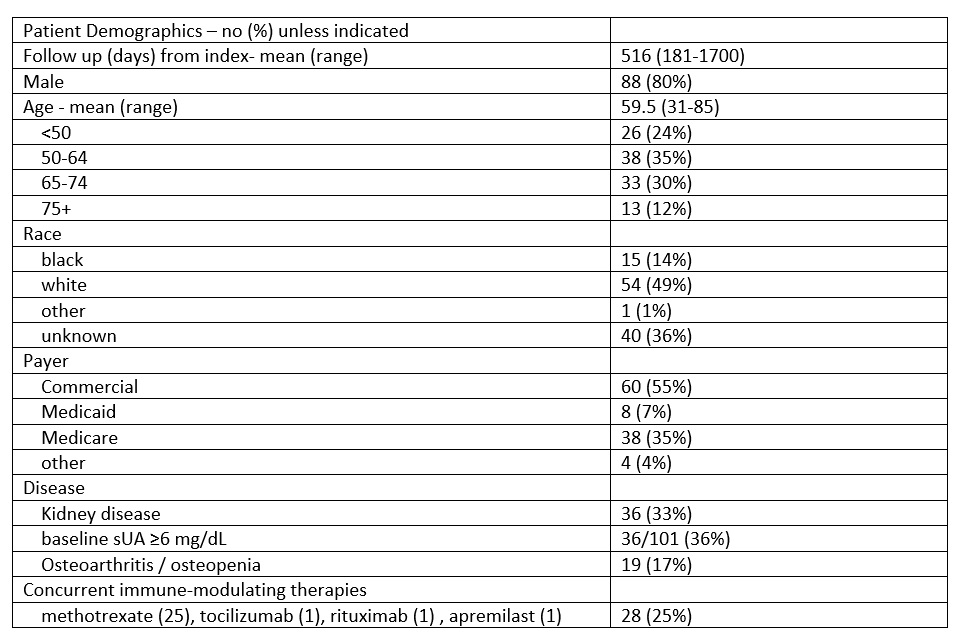 Table 1: Study population characteristics
Table 1: Study population characteristics
 Figure 1: Time to unplanned pegloticase discontinuation stratified by uncontrolled (2+ sUA ≥ 6 mg/dL , green, n=28) or controlled ( < 2 sUA ≥ 6 mg/dL, blue, n=63) sUA
Figure 1: Time to unplanned pegloticase discontinuation stratified by uncontrolled (2+ sUA ≥ 6 mg/dL , green, n=28) or controlled ( < 2 sUA ≥ 6 mg/dL, blue, n=63) sUA
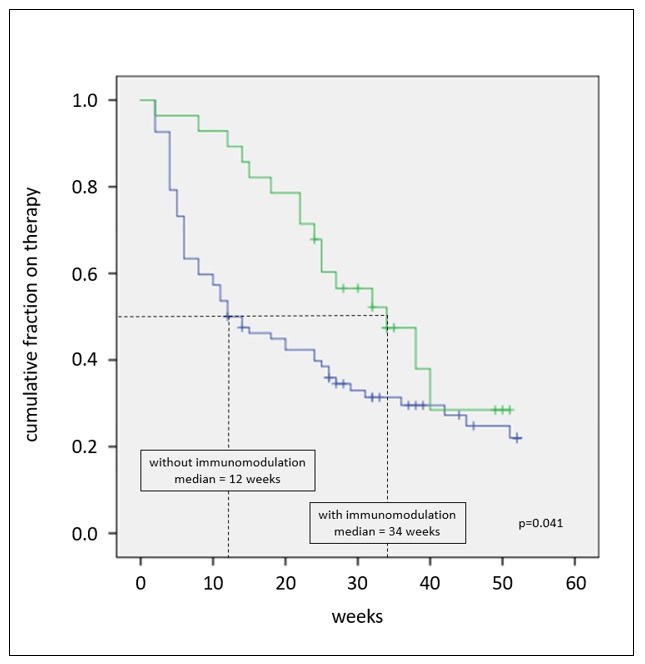 Figure 2: Time to unplanned pegloticase discontinuation stratified by presence (green, n=28) or absence (blue, n=82) of concurrent immune-modulating therapies
Figure 2: Time to unplanned pegloticase discontinuation stratified by presence (green, n=28) or absence (blue, n=82) of concurrent immune-modulating therapies
To cite this abstract in AMA style:
Soloman N, Amin M, Cox K, Helfgott S, Hu A, Huston K, Leonard J, Milligan S, Singh J, Tesser J, Edgerton C. Management of Gout with Pegloticase; Real-World Utilization and Outcomes from Trio Health and the American Rheumatology Network (ARN) [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/management-of-gout-with-pegloticase-real-world-utilization-and-outcomes-from-trio-health-and-the-american-rheumatology-network-arn/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/management-of-gout-with-pegloticase-real-world-utilization-and-outcomes-from-trio-health-and-the-american-rheumatology-network-arn/
