Session Information
Date: Saturday, November 16, 2024
Title: RA – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Few real-life long-term data are available in RA patients initiating tocilizumab (TCZ) depending on age. The objective of this study was to compare the characteristics and management of patients over or under 75 years of age (geriatric age, with no data in RCT).
Methods: Patients having initiated TCZ (IV or SC) with a RA diagnosis in the SNDS (French national health-insurance database, 67 million of people) with 1st date of delivery of TCZ between Jan. 2015 and Dec. 2016 (index date) were included. The end of follow-up date was the study end date (Dec. 2019), the date of death or the date of last reimbursement in the database. Medical history and comorbidities in the 4 years before the index date are reported. A therapeutic line was defined by maintained DMARD deliveries without change such as prolonged ( >90 days) or permanent cessation, switch to another MoA or addition of csDMARD. For tolerance the comparison was made using an adjusted Cox proportional-hazard model with time dependent variables to consider the change of lines of treatments (1st event is considered). aHRs (95% CI) use < 75 years old as reference.
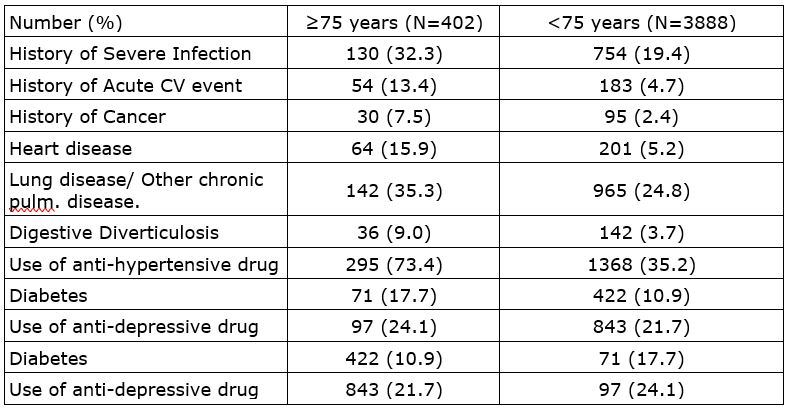
Results: 4290 patients treated with TCZ were included, among them 402 were ≥75 years old (77.6% women). For those ≥75 years old, TCZ was administered SC in 48.5% of the patients, without csDMARD in 66.2% (< 75 years: 58.1% and 51.2% respectively). 83.3% of the patients were on steroids (< 75 years: 76.1%). 69.9% of ≥75 years old had previously received any DMARD (<75 years: 83.6%). Medical history and comorbidities are detailed in the table.
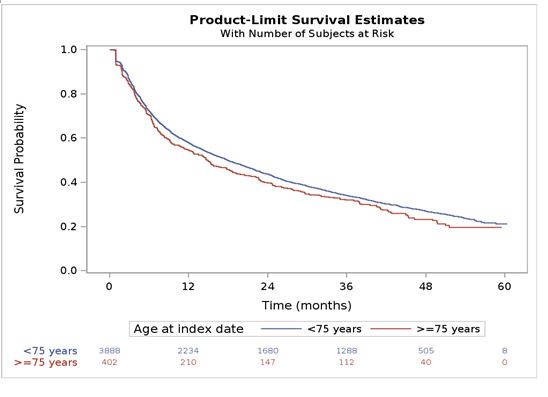
In ≥75 years old, therapeutic maintenance of TCZ was 54%, 39%, 32%, and 23 % at 1, 2, 3 and 4 years. It was 57%, 43%, 34% and 26% among < 75 years old patients respectively (Fig.). The median duration of TCZ was 15 months 95%CI (11.6-18.6) in patients ≥75 years old and 18 months 95%CI (16.6-19.5) in < 75 years old.
55.5% of ≥75 years old and 20.4% of < 75 years old had received an influenza vaccine within the year. 42% of ≥75 years old and 53.9% of < 75 years old received an anti-pneumococcal vaccine within 4 years before index date. At 5 years, patients ≥75 years old had had 188 episodes of severe infections [47.6% of patients, aHR:1.80 (1.53-2.13)], 73 acute CV events [18.1%, aHR 2.13 (1.60-2.83)], 41 digestive perforations [10.1%, aHR 1.32 (0.92-1.88)], 27 hematological events [6.7%, aHR 1.89 (1.22-2.91)] and 17 cancers [4.2%, aHR 1.06 (0.62-1.79)]. 161 patients died, including 67 of the ≥75 years old.
Conclusion: This analysis shows that TCZ can be a relevant option in older RA patients with therapeutic maintenance close to that of younger patients. However, patient monitoring should be carefully followed-up since these patients are more likely to develop adverse events.
To cite this abstract in AMA style:
Fautrel B, SARAUX A, Idier I, Bonnabau H, Bizouard G, COMBE B. Management of Elederly Patients with Rheumatoid Arthritis Treated with Tocilizumab : Comparison of Patients over and Under 75 Years Old [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/management-of-elederly-patients-with-rheumatoid-arthritis-treated-with-tocilizumab-comparison-of-patients-over-and-under-75-years-old/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/management-of-elederly-patients-with-rheumatoid-arthritis-treated-with-tocilizumab-comparison-of-patients-over-and-under-75-years-old/