Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Ocular manifestations are common in ANCA-associated vasculitides (AAV), with orbital inflammatory disease (OID) representing a significant subset. Despite being a vision-threatening complication, OID remains a significant therapeutic challenge. The aim of this systematic review was to evaluate the efficacy and safety of interventions for AAV-associated OID.
Methods: We searched for clinical trials, case-control studies, observational studies and case series ( >=5 patients) of adult patients undergoing therapy for AAV-associated OID within Embase Classic + Embase, MEDLINE(R) via Ovid, Web of Science Core Collection, Clinicaltrials.gov, and Cochrane Central Register of Controlled Trials without language or date restrictions. Included studies were required to clearly report on types and dosing of therapies used and clinical response after at least 1 month of follow up or later. The primary outcome was clinical response, defined as any improvement in signs and/or symptoms, up to and including the achievement of clinical remission (complete response). Secondary outcomes included clinical remission, relapse, sustained remission ( >6 months) and serious adverse events. Two reviewers independently screened for relevant records and critically appraised included articles using the Newcastle Ottawa Scale (NoS) checklist. We performed a qualitative synthesis of results from eligible articles. We also performed a meta-analysis of proportions for any outcomes with sufficient clinical homogeneity using a random effects model and restricted maximum likelihood approach. Analyses were conducted using Stata SE (v18.0).
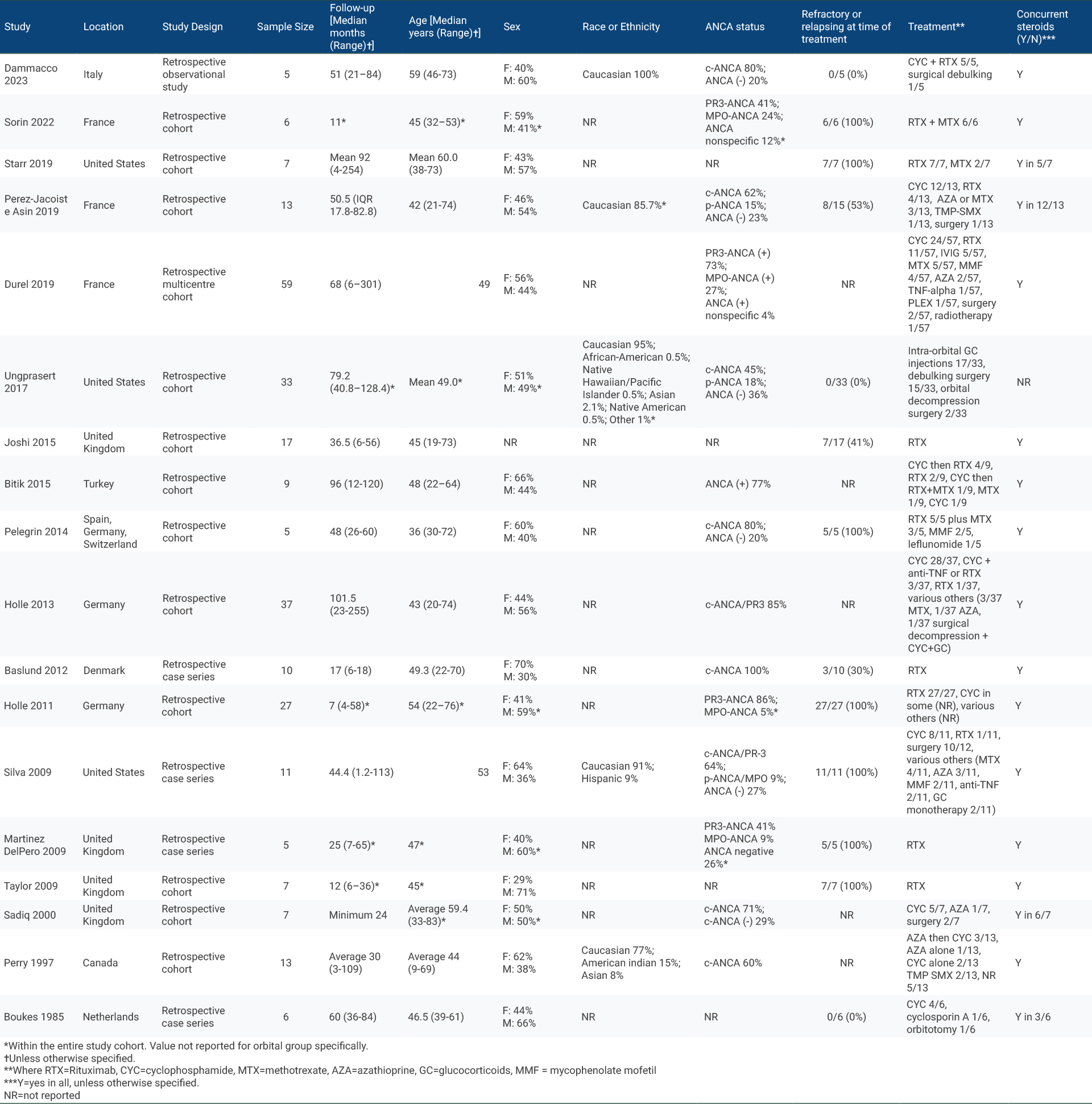
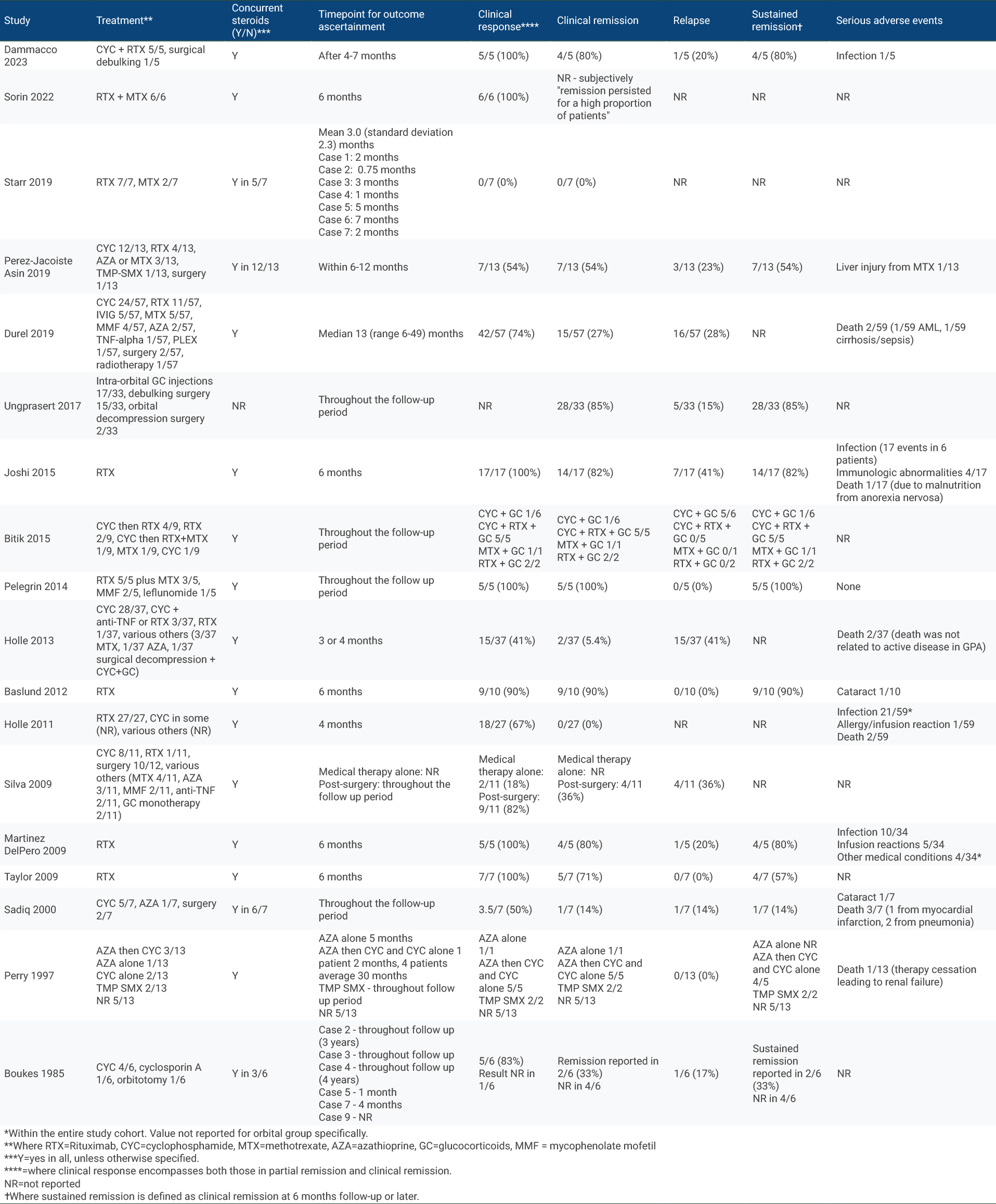
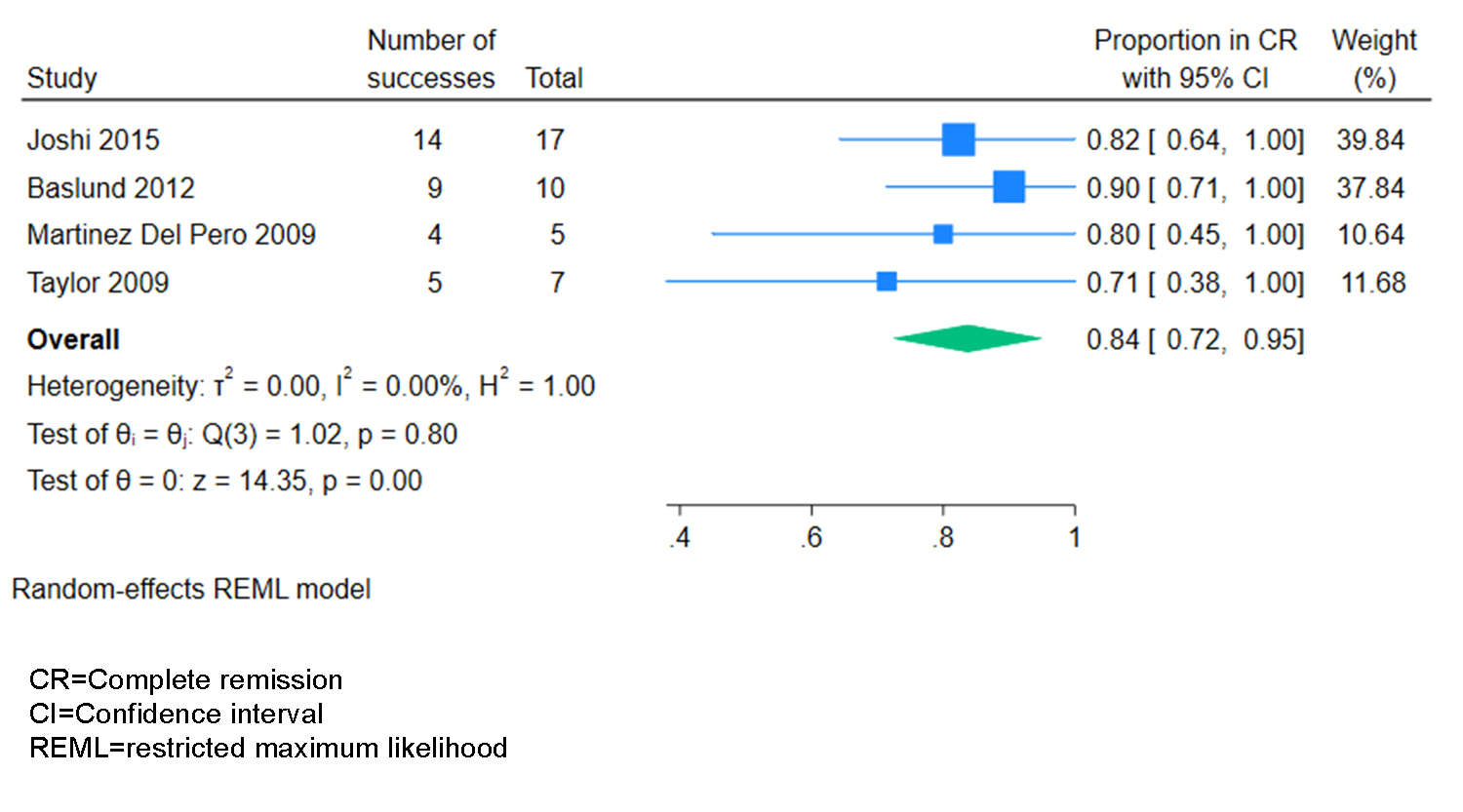
Results: The search strategy identified 1001 unique studies. After title and abstract screening, we included 119 (12%) in the full-text review, of which 18 (1.8%) met eligibility criteria and were included, representing 277 patients (Table 1). 17/18 studies had NoS scores of 5 or higher. Study designs included retrospective cohort (n=14) and case series (n=4). Treatment regimens varied across and within studies: 13 studies included patients who received rituximab (RTX), 10 studies included patients who received cyclophosphamide (CYC), 7 studies included patients who received RTX plus another immunosuppressant (IS), 11 studies included patients who received conventional IS, and 7 studies included patients who underwent surgery. The most frequent time point for reporting treatment outcomes was 6 months (5 studies, 4 of RTX and 1 of RTX + IS) (Table 2). Of these, all studies (5/5) observed some clinical response in 100% of individuals. With RTX, clinical remission/complete response at 6 months was achieved in 84% of individuals (95% CI 72%-95%, z=14.35, p< 0.001) (Figure 1).
Conclusion: Studies have reported a variety of therapies for AAV-associated OID. Pooled estimates showed that over three quarters of individuals achieved clinical remission with RTX at 6 months, and qualitatively, high rates of remission were reported for RTX + conventional IS. Our results emphasize the necessity of larger, prospective studies examining treatments for AAV-associated OID.
To cite this abstract in AMA style:
Neary E, Healey K, Clements-Baker M, Makhzoum J, Mendel A. Management of ANCA Vasculitis-Associated Orbital Inflammatory Disease: A Systematic Literature Review [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/management-of-anca-vasculitis-associated-orbital-inflammatory-disease-a-systematic-literature-review/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/management-of-anca-vasculitis-associated-orbital-inflammatory-disease-a-systematic-literature-review/