Session Information
Date: Monday, November 14, 2022
Title: RA – Treatment Poster IV
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Patients with IMID, and notably patients with rheumatoid arthritis RA, are at increased risk of cancer compared with the general population. It is hence paramount to assess the impact of biological or targeted DMARD (e.g.., tofacitinib and TNFi) on the risk of cancer outcome in patients already at-risk, particularly in the context of ORAL Surveillance which showed a higher risk for malignancies (excluding NMSC) with tofacitinib, in comparison with TNFI, in RA patients
Methods: The RELATION study is a retrospective observational cohort study using the French nationwide healthcare database (SNDS). Patients aged 18 years or older, affiliated to the French national health insurance with a diagnosis of RA and initiating tofacitinib after November 1, 2017 or TNFi after January 1, 2010 (including adalimumab, etanercept, or other TNFi, without previous exposure to tofacitinib) were followed from treatment initiation to December 31, 2020. Patients with a previous history of a malignancy (excluding nonmelanoma skin cancer, NMSC) in the 4 years preceding cohort entry were excluded. All malignancies events were defined by the first hospitalization for malignancy using ICD-10 codes during follow-up. Comorbidities and traditional cardiovascular (CV) risk factors were identified using hospitalizations, procedures, or medication dispensing in the 4 years prior cohort entry. The unadjusted incidence rate (IR) of malignancies (excluding NMSC) was assessed in patients initiating either tofacitinib or a TNFi. The associated 95% confidence intervals (95% CI) were calculated using the exact Poisson distribution and were two-sided.
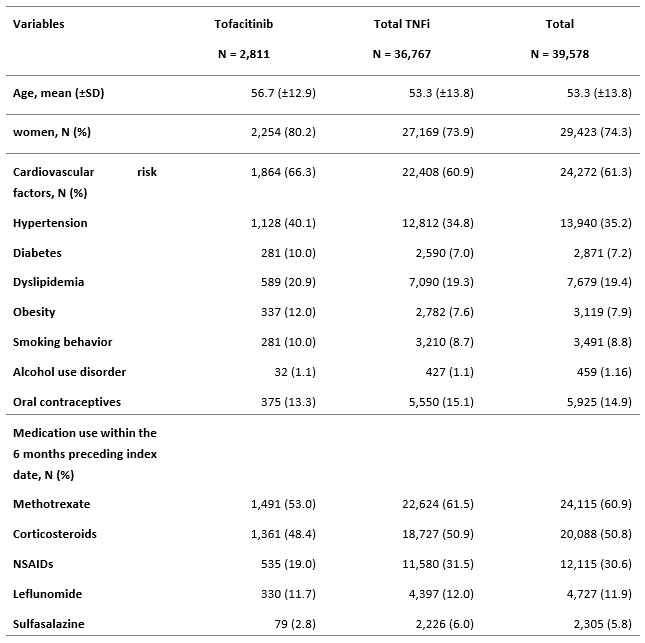
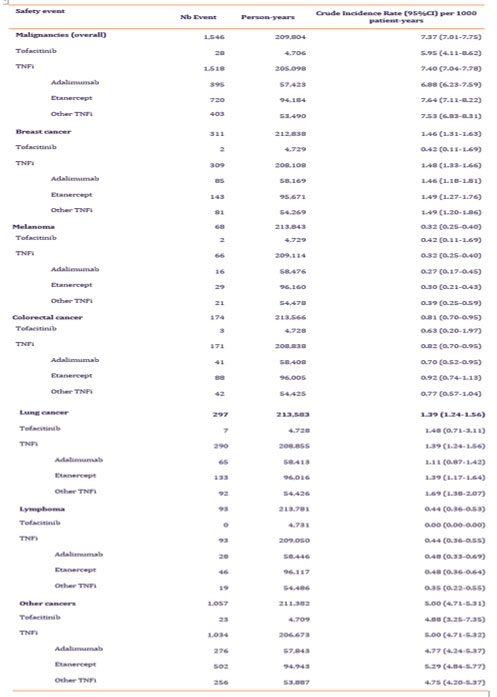
Results: Between 2010 and 2020, a total of 39,578 patients with RA were included in the study. Among these, 2,811 initiated tofacitinib and 36,767 initiated a TNFi (adalimumab: 10,621, etanercept: 16,512, other TNFi: 9,634). Patients had a mean age of 53 years at cohort entry, and 72% to 81% were women. Around 61% of the cohort had at least one cardiovascular CV risk factor, arterial hypertension being the most frequent (35.2%). The proportion of patient with CV risk factor was numerically higher in the tofacitinib group. The two major co-medications at treatment initiation in the two groups were methotrexate (60.9%) and corticosteroids (50.8%) (table 1). 28 incident malignancy events occurred in the tofacitinib group (IR: 5.9 (4.1-8.6) per 1,000 patient-year (PY)) and 1,518 occurred in the TNFi group (IR: 7.4 (7.0-7.8) per 1,000 PY). The IR per 1000 PY of breast cancer, colorectal cancer and lung cancer were respectively 0.42 (0.11-1.69), 0.63 (0.20-1.97) and 1.48 (0.71-3.11) in the tofacitinib group and 1.48 (1.33-1.66), 0.82 (0.70-0.95) and 1.39 (1.24-1.56) in the TNFi group. No incident lymphoma occurred in tofacitinib-users, and it affected patients taking TNFi with an IR of 0.44 (0.36-0.55), The IR of other cancers was 4.9 (3.2-7.4) and 5.0 (4.7-5.3) per 1,000 PY in the tofacitinib and the TNFi groups respectively (Table 2).
Conclusion: This study assessed malignancies occurrence in RA patients initiating tofacitinib or TNFi in a French nationwide setting. Adjusted comparative results will allow to compare this occurrence between the different groups of interest.
To cite this abstract in AMA style:
gottenberg j, Mammar N, Kessouri M, RUDANT J, Assi n, raguideau F, kirchgesner j. Malignancies Risk in Rheumatoid Arthritis Patients Treated with Tofacitinib or TNF Inhibitors, a National Study: RELATION Study [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/malignancies-risk-in-rheumatoid-arthritis-patients-treated-with-tofacitinib-or-tnf-inhibitors-a-national-study-relation-study/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/malignancies-risk-in-rheumatoid-arthritis-patients-treated-with-tofacitinib-or-tnf-inhibitors-a-national-study-relation-study/