Session Information
Date: Sunday, November 17, 2024
Title: RA – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: FILOSOPHY (NCT04871919) and PARROTFISH (NCT05323591) are ongoing, prospective, observational Phase 4 studies of filgotinib in patients with RA. This interim analysis evaluated effectiveness and PROs by prior biological (b) DMARD use, work productivity and safety.
Methods: FILOSOPHY includes adults with moderate to severe active RA treated with filgotinib for the first time in daily practice. In this analysis DAS28-CRP, CDAI, pain (visual analog scale [VAS]), FACIT-Fatigue and HAQ-DI were assessed (observed cases) in patients who were bDMARD naïve and who had received 1 or ≥2 prior bDMARDs; these subgroups excluded patients who had received prior targeted synthetic (ts) DMARDs. In all patients, work productivity was assessed with the Work Productivity and Activity Impairment (WPAI) questionnaire, and treatment-emergent adverse events (TEAEs) on study were recorded.
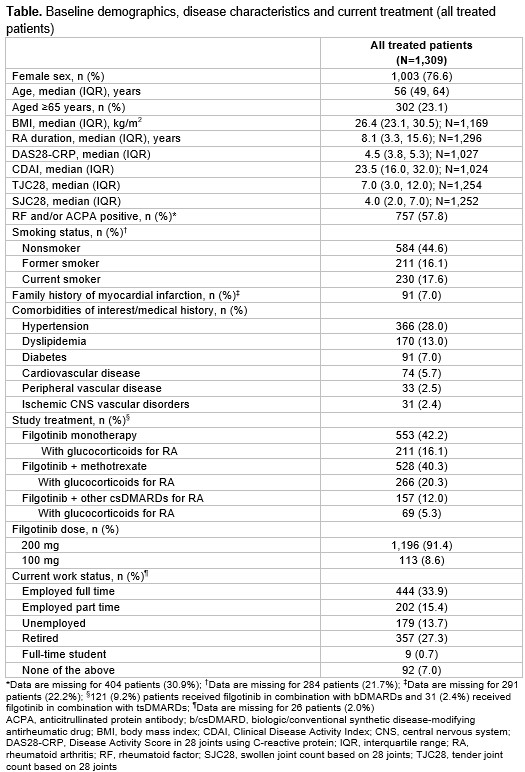
Results: This interim analysis includes 1,309 patients treated from May 2021 to Jan 2024, (median follow-up 366 days). 7.4% of patients had completed 2 years of follow-up. Baseline characteristics are shown in Table. The ‘bDMARD naïve’, ‘1 prior bDMARD’ and ‘≥2 prior bDMARDs’ subgroups (all tsDMARD naive) comprised 37.1% (n=485), 23.2% (n=304) and 23.1% (n=302) of patients, respectively. The remaining 218 patients (16.7%) had received prior tsDMARDs.
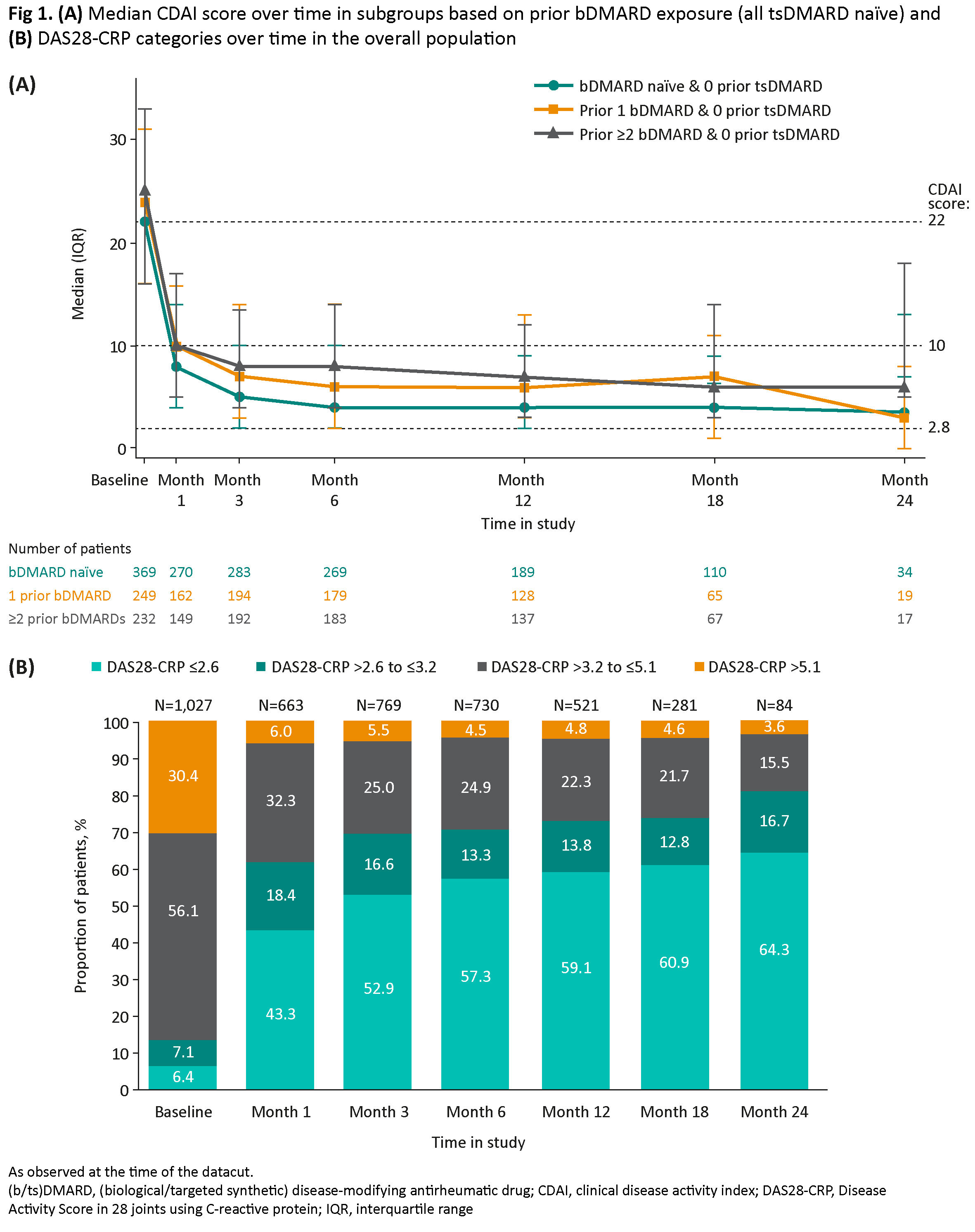
Median CDAI (Fig 1A) and DAS28-CRP decreased from baseline to Month 1 in all subgroups and remained stable to Month 24. Overall, DAS28-CRP ≤3.2 was achieved by 70.5% (515/730) of patients at Month 6 and 81.0% (68/84) at Month 24 (Fig 1B); CDAI ≤10 was achieved by 67.0% (511/763) of patients at Month 6 and 77.5% (69/89) at Month 24.
In the ‘bDMARD naïve’, ‘1 prior bDMARD’ and ‘≥2 prior bDMARDs’ subgroups, VAS pain and FACIT-Fatigue improved from Week 1, with improvements maintained to Month 24. In each subgroup, HAQ-DI improved from Month 1, with improvements maintained to Month 12.
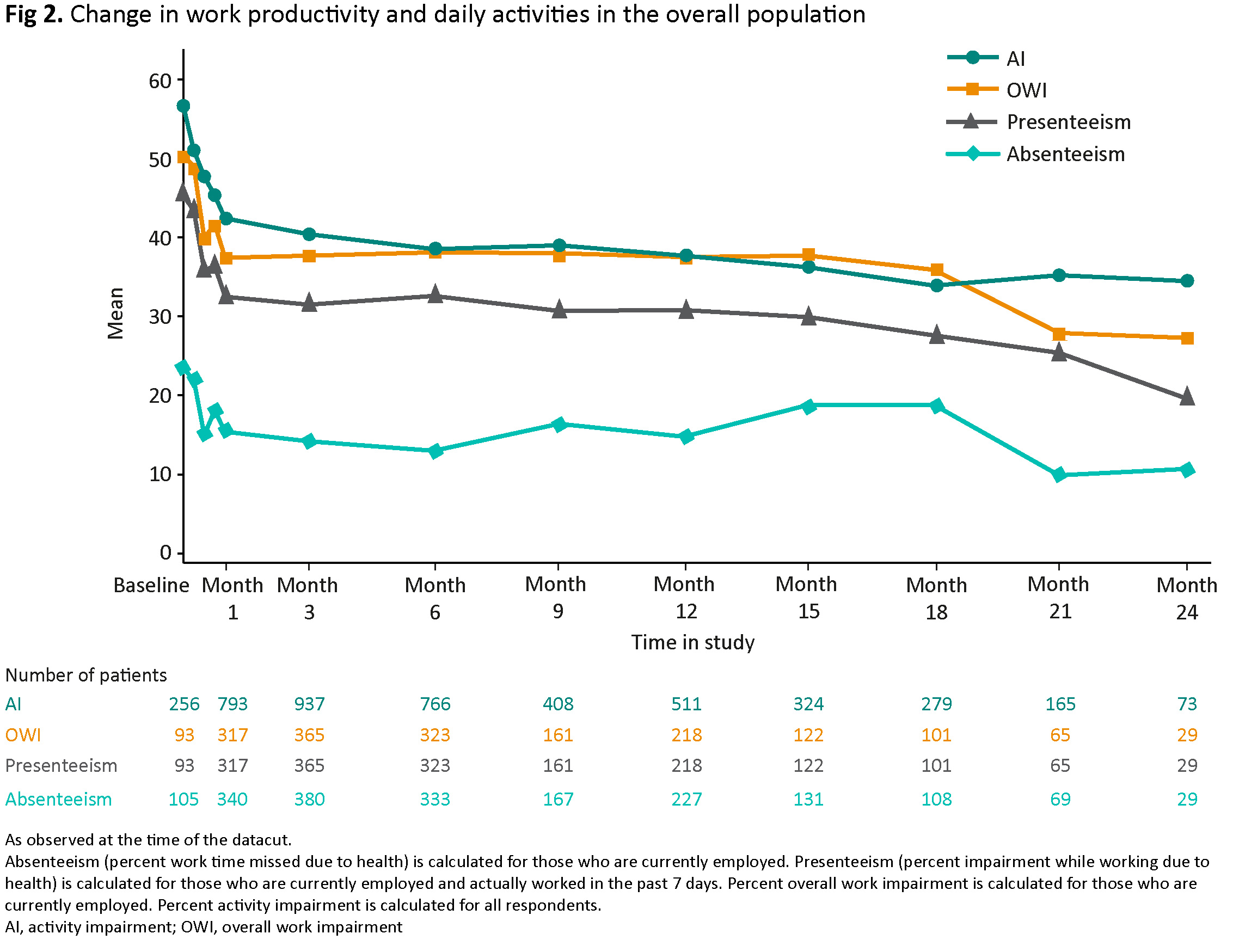
Overall, WPAI parameters improved by Week 1, with further improvements until Month 24. Mean (SD) activity impairment decreased from 56.6 (23.7) at baseline (N=256) to 42.4 (26.1) at Month 1 (N=793) and 34.5 (27.3) at Month 24 (N=73) (Fig 2).
Overall, 700 patients (53.5%) had a TEAE, leading to treatment discontinuation in 127 (9.7%). There were 6 deaths; 1 was considered treatment related. The exposure-adjusted incidence rates for TEAEs of interest per 100 patient-years (95% CI) were: COVID-19 11.1 (9.2, 13.2); opportunistic infections 0.3 (0.1, 0.7); herpes zoster 1.7 (1.0, 2.6); fractures 1.8 (1.1, 2.7); malignant/unspecified tumor 0.9 (0.5, 1.7); unstable angina 0.7 (0.3, 1.3); stroke 0.5 (0.2, 1.1); cardiac failure 0.1 (0.0, 0.5); transient ischemic attack 0.4 (0.1, 1.0); and pulmonary embolism 0.3 (0.1, 0.7).
Conclusion: In this interim analysis, filgotinib improved disease activity as early as Month 1, with approx. 80% of patients achieving low disease activity (DAS28-CRP or CDAI) by Month 24. Pain and fatigue improved as early as Week 1 and HAQ-DI as early as Month 1, with improvements maintained to Month 24 (Month 12 for HAQ-DI). Improvements in disease activity and PROs were observed irrespective of prior bDMARD exposure. In the overall population, work productivity parameters improved and there were no new safety signals.
To cite this abstract in AMA style:
Galloway J, Avouac J, Burmester G, Caporali R, Debray T, Van Beneden K, Betteridge N, Romero Yuste S, Zignani M, Verschueren P, Bevers K. Maintained Improvement of Disease Activity and Patient-reported Outcomes (PROs) with Filgotinib in Patients with Rheumatoid Arthritis (RA) in the Real World: Up to 2-year Interim Data from FILOSOPHY [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/maintained-improvement-of-disease-activity-and-patient-reported-outcomes-pros-with-filgotinib-in-patients-with-rheumatoid-arthritis-ra-in-the-real-world-up-to-2-year-interim-data-from-filosophy/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/maintained-improvement-of-disease-activity-and-patient-reported-outcomes-pros-with-filgotinib-in-patients-with-rheumatoid-arthritis-ra-in-the-real-world-up-to-2-year-interim-data-from-filosophy/