Session Information
Date: Saturday, November 12, 2022
Title: Abstracts: Miscellaneous Rheumatic and Inflammatory Diseases I
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Patients with untreated immune-mediated inflammatory diseases, such as RA, PsA, and AS, are at increased risk for major adverse cardiovascular events (MACE) and venous thromboembolic events (VTE) compared with the general population.
The purpose of this analysis was to describe the events and risk factors for MACE and VTE in the RA, PsA, and AS clinical trial programs of the Janus kinase (JAK) inhibitor, upadacitinib (UPA).
Methods: Treatment-emergent adverse events (TEAEs) of MACE and VTE from 9 (6 RA; 2 PsA; 1 AS) pivotal, randomized, controlled, phase 2b/3 trials were summarized for UPA 15 mg (approved rheumatology dose), UPA 30 mg, adalimumab (ADA) 40 mg, and MTX. TEAEs were defined as onset on or after the first dose of study drug and up to 30 or 70 days after last dose for UPA/MTX or ADA, respectively. TEAEs of MACE (cardiovascular [CV] death, non-fatal myocardial infarction, and non-fatal stroke) and VTE (pulmonary embolism [PE] and deep vein thrombosis [DVT]) were adjudicated by a blinded, independent cardiovascular adjudication committee. Patients were not censored at the time of event; data are presented as exposure adjusted event rates (EAERs) in events per 100 patient-years (E/100 PY), with a cutoff date of 30 June 2021. Time-to-event was analyzed using the Kaplan-Meier method, and EAERs were evaluated over 6-month increments. Given the limited number of events, Cox-regression were limited to univariable analyses and assessed the relationship between potential risk factors and occurrence of MACE and VTE in patients receiving UPA.
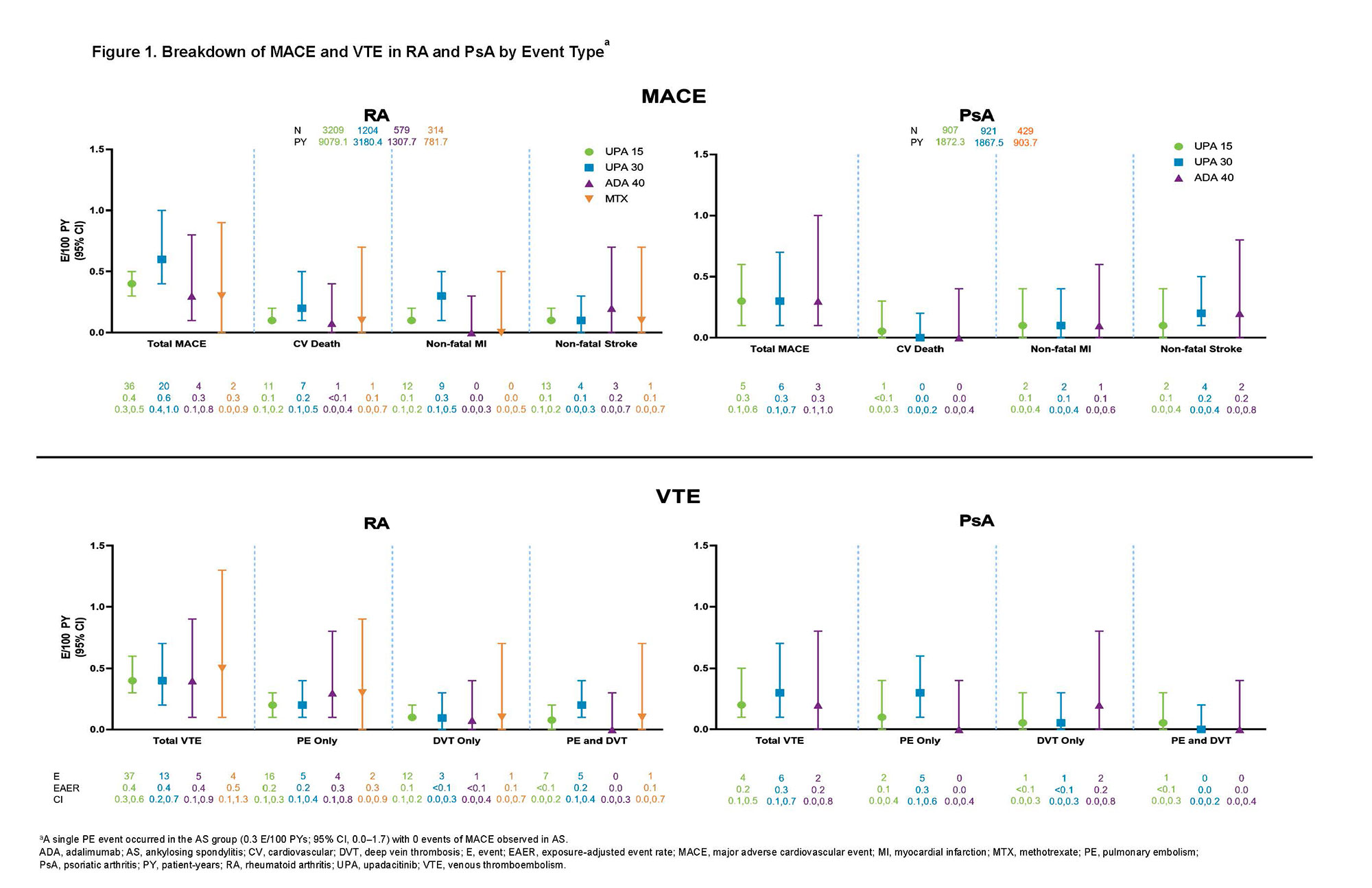
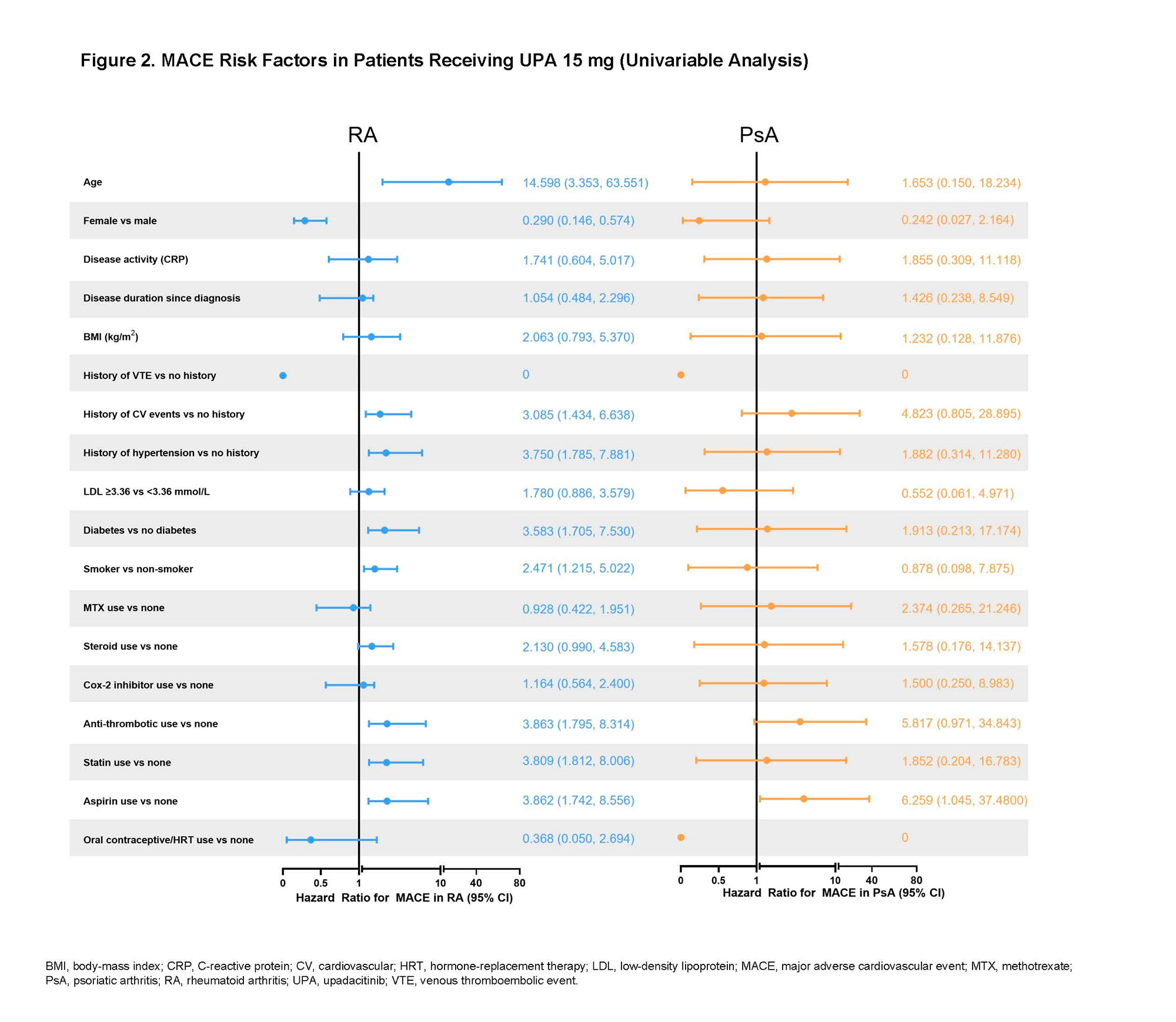
Results: Across trials, 4298 patients received ≥ 1 dose of UPA 15 mg and 2125 received UPA 30 mg, 1008 patients received ADA 40 mg, and 314 patients received MTX. At baseline, 40%–50% of patients had 2 or more CV risk factors, and the proportion of patients ≥ 65 years ranged from 6%–23%. EAERs of MACE and VTE in RA and PsA are presented in Figure 1, with 0 MACE and 1 VTE (PE) reported in AS. Of the 41 MACE reported in the UPA 15 mg group across RA and PsA, only 2 RA patients did not have ≥ 1 CV risk factors at baseline. There were 2 fatal VTEs across trials, both occurring in the RA UPA 15 mg group. Overlapping confidence intervals were observed across UPA doses and comparators for rates of MACE as well as for rates of VTE in both RA and PsA. There was no pattern of time-to-event of EAERs by 6-month intervals over 42 months observed in patients receiving UPA (data not shown). In univariable analyses, factors potentially associated with MACE or VTE occurrence in patients with RA receiving UPA 15 mg included age ≥ 65 years; baseline hypertension; diabetes mellitus; smoking; history of CV event or VTE; as well as use of aspirin, statins, or antithrombotics. In PsA, aspirin use was associated with increased risk of MACE. The full list of evaluated factors is presented in Figures 2 and 3.
Conclusion: Rates of adjudicated MACE and VTE with UPA were infrequent and consistent with background rates in RA, PsA, and AS populations. The patient characteristics found to be associated with MACE and VTE are known risk factors for these events. Continued follow-up is ongoing to further contextualize the risk of MACE and VTE in the UPA clinical trial programs.
To cite this abstract in AMA style:
Charles-Schoeman C, Choy E, McInnes I, Mysler E, Nash P, Yamaoka K, Lippe R, Khan N, Shmagel A, Palac H, Suboticki J, Curtis J. MACE and VTE Across Upadacitinib Clinical Trial Programs in Rheumatoid Arthritis, Psoriatic Arthritis, and Ankylosing Spondylitis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/mace-and-vte-across-upadacitinib-clinical-trial-programs-in-rheumatoid-arthritis-psoriatic-arthritis-and-ankylosing-spondylitis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/mace-and-vte-across-upadacitinib-clinical-trial-programs-in-rheumatoid-arthritis-psoriatic-arthritis-and-ankylosing-spondylitis/