Session Information
Date: Saturday, November 12, 2022
Title: SLE – Diagnosis, Manifestations, and Outcomes Poster I: Diagnosis
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
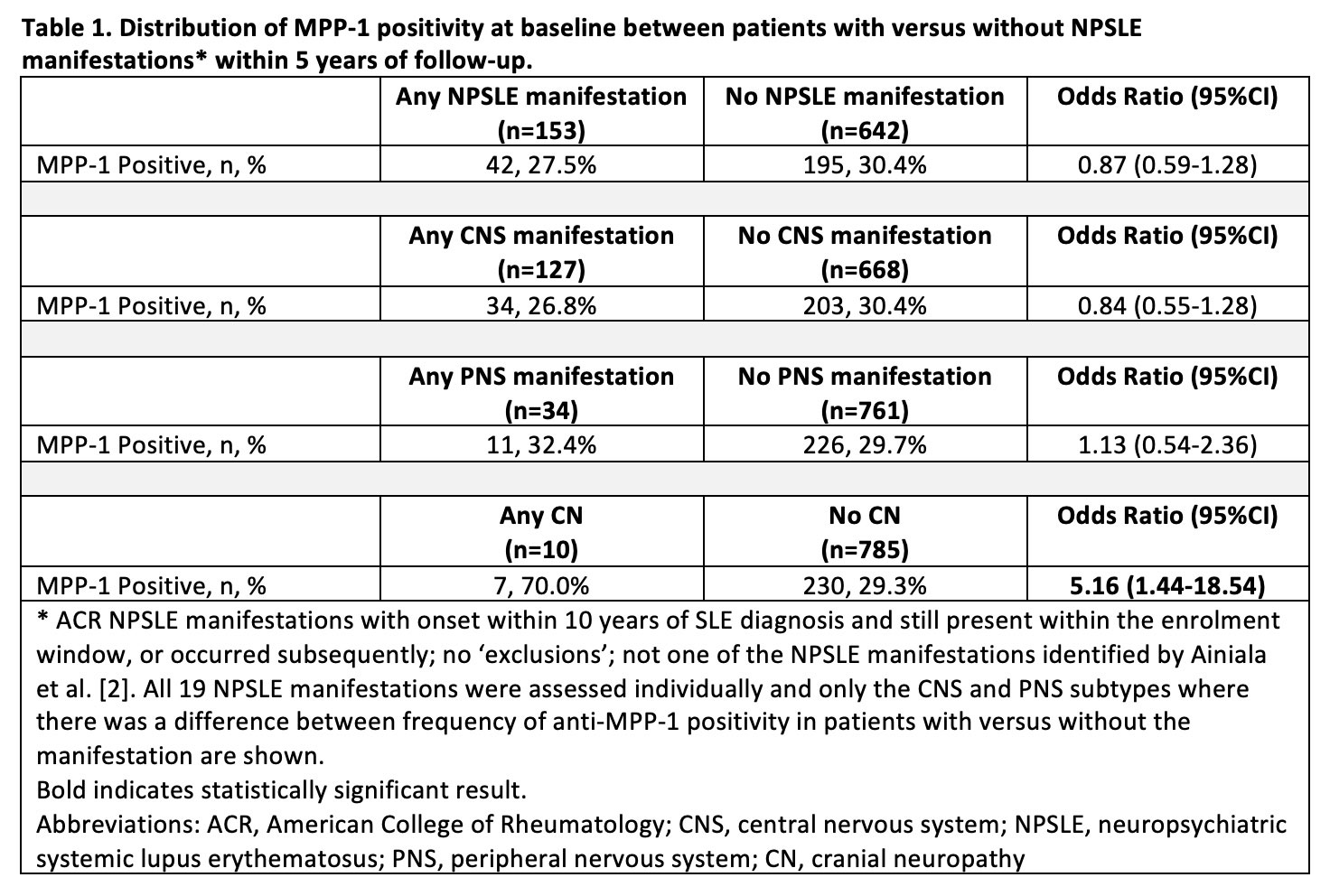
Background/Purpose: We previously reported in a single centre prevalent SLE cohort that antibodies against the cytokinesis-associated protein M-Phase Phosphoprotein 1 (anti-MPP-1) were associated with SLE-related cranial neuropathy (CN), a rare manifestation of neuropsychiatric SLE (NPSLE). The purpose of this study was to assess whether anti-MPP-1 is a biomarker for CN or other NPSLE manifestations using an international SLE inception cohort.
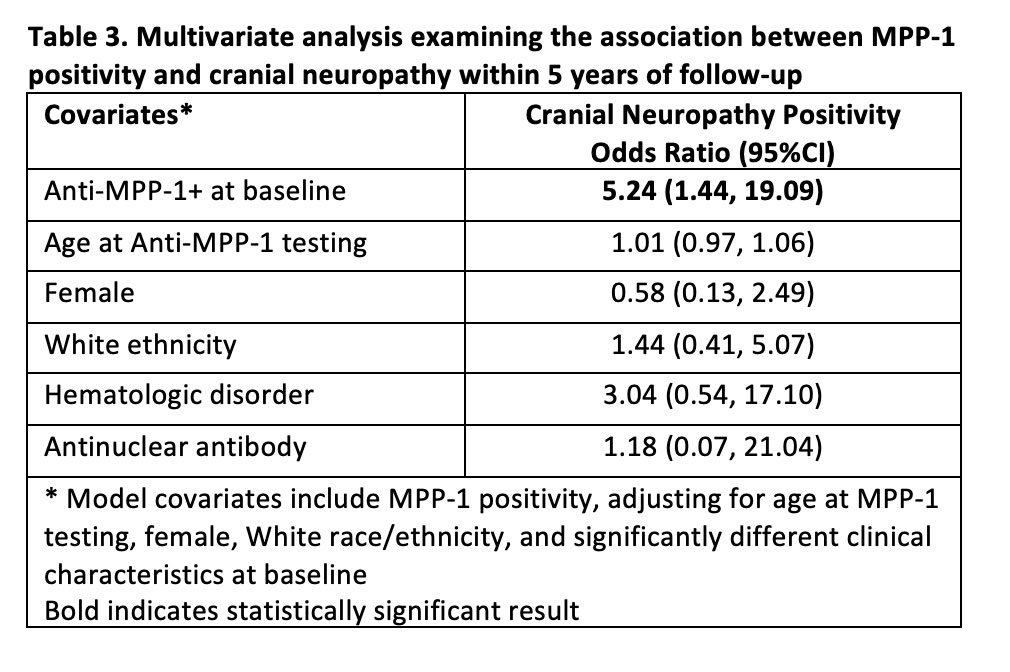
Methods: SLE patients fulfilling the updated 1997 ACR classification criteria for SLE were included. Anti-MPP-1 antibody testing was performed on baseline samples (within 15 months of diagnosis) or first annual assessment using an addressable laser bead immunoassay (ALBIA) with purified recombinant human protein with results expressed as median florescence units (MFU). Based on healthy controls, a dilution of ≥1:500 MFU was considered positive. NPSLE manifestations occurring over the first 5 years of follow up were documented annually based on ACR case definitions using published NPSLE attribution rules [1]). The frequency of anti-MPP-1 positivity between patients with versus without each of the 19 NPSLE manifestations was compared using univariate logistic regression. For any NPSLE manifestations where anti-MPP-1 positivity differed between patients with versus without the manifestation, baseline demographic and clinical characteristics were compared using T-tests and two-sample tests of proportions. For NPSLE manifestations associated with anti-MPP-1 positivity in the univariate analysis, multivariable logistic regression analysis using penalized maximum likelihood estimates was then performed to assess the relationship between anti-MPP-1 and the NPSLE manifestation, adjusting for age at anti-MPP-1 testing, female, White race/ethnicity, and significantly different baseline clinical characteristics.
Results: Seven hundred and ninety-five SLE patients with complete data for 5 years of follow up were assessed; 29.8% were anti-MPP1 positive, 88.7% female, and 52.1% White. The frequency of anti-MPP-1 positivity differed only for those with versus without CN (70.0% vs. 29.3%; odds ratio [OR] 5.16, 95%CI 1.44, 18.54) (Table 1). Compared to patients without CN (n=785), patients with CN (n=10) were more likely to fulfill the ACR hematologic (difference: 23.9%, 95%CI 5.0%, 42.8%) and antinuclear antibody criteria (difference: 4.3%, 95%CI 2.9%, 5.8%) (Table 2). In the multivariate analysis, anti-MPP1 remained associated with CN (OR 5.24, 95%CI 1.44, 19.09) after adjusting for age at anti-MPP-1 testing, female, White race/ethnicity, hematologic disorder, and antinuclear antibody (Table 3).
Conclusion: Anti-MPP-1 is a potential biomarker for CN in SLE. Although anti-MPP-1 is differentially expressed in a variety of neurological cells and tissues, the link to a pathogenic role requires further study.
References:
1. Hanly, J. G., Urowitz, M. B., Gordon, C., et al. Ann Rheum Dis. 2020; 79(3): 356-362.
2. Ainiala H , Hietaharju A , Loukkola J , et al . Arthritis Rheum 2001 ;45: 419–23.
To cite this abstract in AMA style:
Krustev E, Hanly J, Chin R, Buhler K, Cardwell F, Urowitz M, Gordon C, Bae S, Romero-Diaz J, Sanchez-Guerrero J, Bernatsky S, Wallace D, Isenberg D, Rahman A, Merrill J, Fortin P, Gladman D, Bruce I, Petri M, Ginzler E, Dooley M, Ramsey-Goldman R, Manzi S, Jönsen A, Alarcón G, van Vollenhoven R, Aranow C, Mackay M, Ruiz-Irastorza G, Lim S, İnanç M, Kalunian K, Jacobsen S, Peschken C, Kamen D, Askanase A, Buyon J, Fritzler M, Clarke A, Choi M. M-Phase Phosphoprotein 1 (MPP-1) Autoantibodies as a Potential Biomarker for Cranial Neuropathies in an International SLE Inception Cohort [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/m-phase-phosphoprotein-1-mpp-1-autoantibodies-as-a-potential-biomarker-for-cranial-neuropathies-in-an-international-sle-inception-cohort/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/m-phase-phosphoprotein-1-mpp-1-autoantibodies-as-a-potential-biomarker-for-cranial-neuropathies-in-an-international-sle-inception-cohort/