Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose:
Impaired clearance of cell debris allows nuclear self-antigens such as DNA to accumulate, bind autoreactive IgG and form immune complexes (IgG-ICs) in SLE. We recently reported that multiple models of lupus-prone mice harbor defects in lysosome acidification. Reduced acidification diminishes the degradation of IgG-ICs and induces their recycling back to the plasma membrane. This perpetuates FcgR signal transduction which in turn further impairs lysosome function, and promotes BAFF secretion. The sustained presence of IgG-ICs in the endocytic pathway causes phagolysosome membrane permeability allowing IgG-ICs to enter the cytosol, activating Type I IFN and inducing pyroptosis. In this study, we translate our murine findings and assess whether lysosomal defects and surface accumulation of DNA-containing ICs are evident in active vs inactive SLE patients.
Methods:
We enrolled ANA positive active or inactive SLE patients (SLEDAI ≥ 6, or £ 5 meeting 2012 SLICC criteria) and seropositive (RF/CCP) active rheumatoid arthritis (RA) patients (2010 ACR criteria and DAS28 >5.1). Healthy controls (HCs) were enrolled from a platelet donation center.
The levels of surface-bound antigen (DNA or CCP) from circulating hematopoietic cells (monocytes, dendritic cells, T and B cells), in SLE, RA and HC were analyzed by flow cytometry. Lysosomal pH was measured using ratiometric flow cytometry from whole blood cells treated with exogenous IgG-ICs. Lysosomal pH changes were normalized to cells treated with Concanamycin A, an inhibitor of lysosome acidification.
Results:
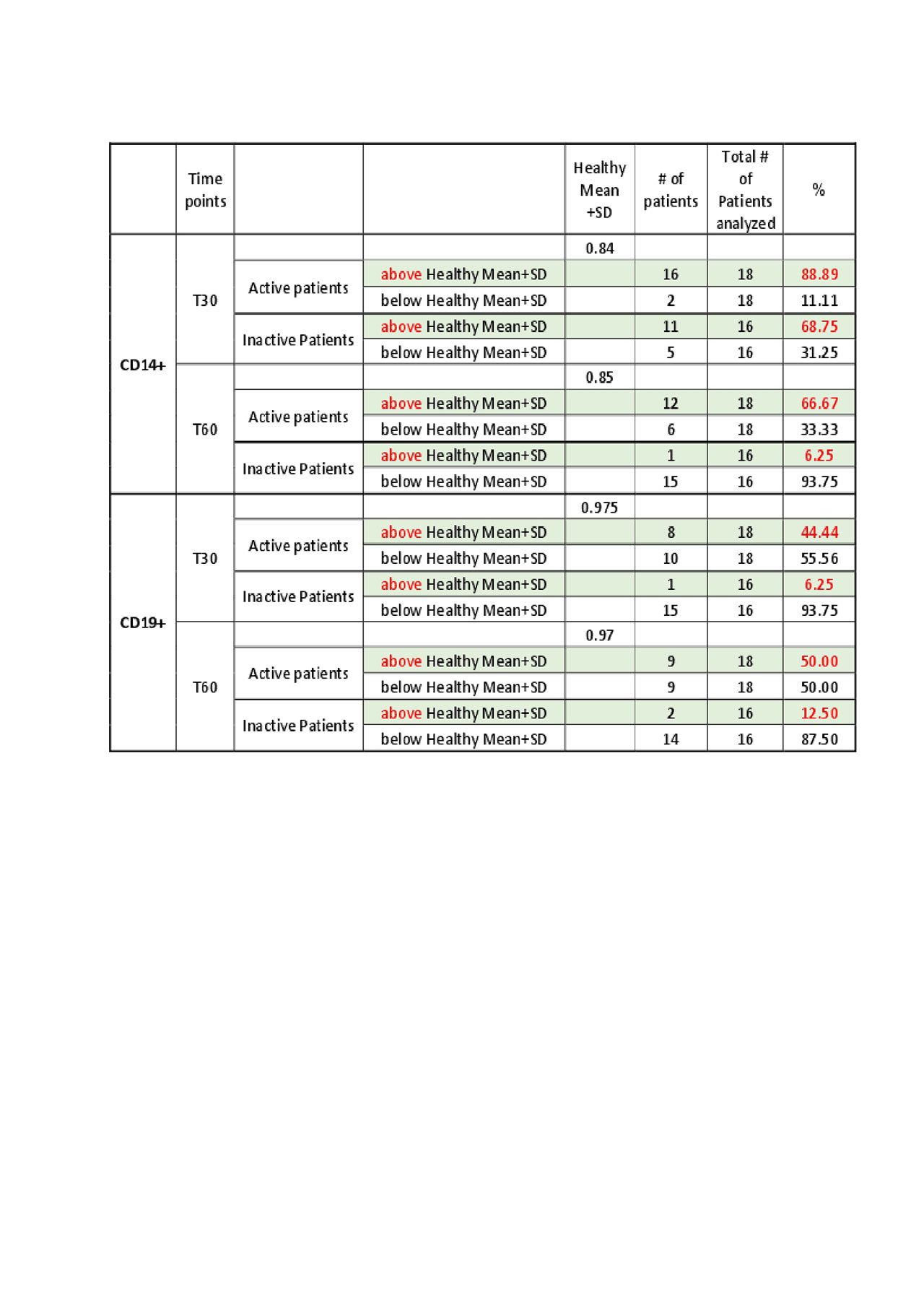
We measured relative lysosome pH in blood monocytes and B cells from 18 active and 16 inactive SLE patients with 16 HCs. Among active SLE patients, 67% of monocytes and 50% of B cells showed defective pH changes at 60 min (relative pH greater than mean+SD of HC). Of inactive SLE patients, only 6% of monocytes and 13% of B cells showed defective lysosomal acidification (Table 1).
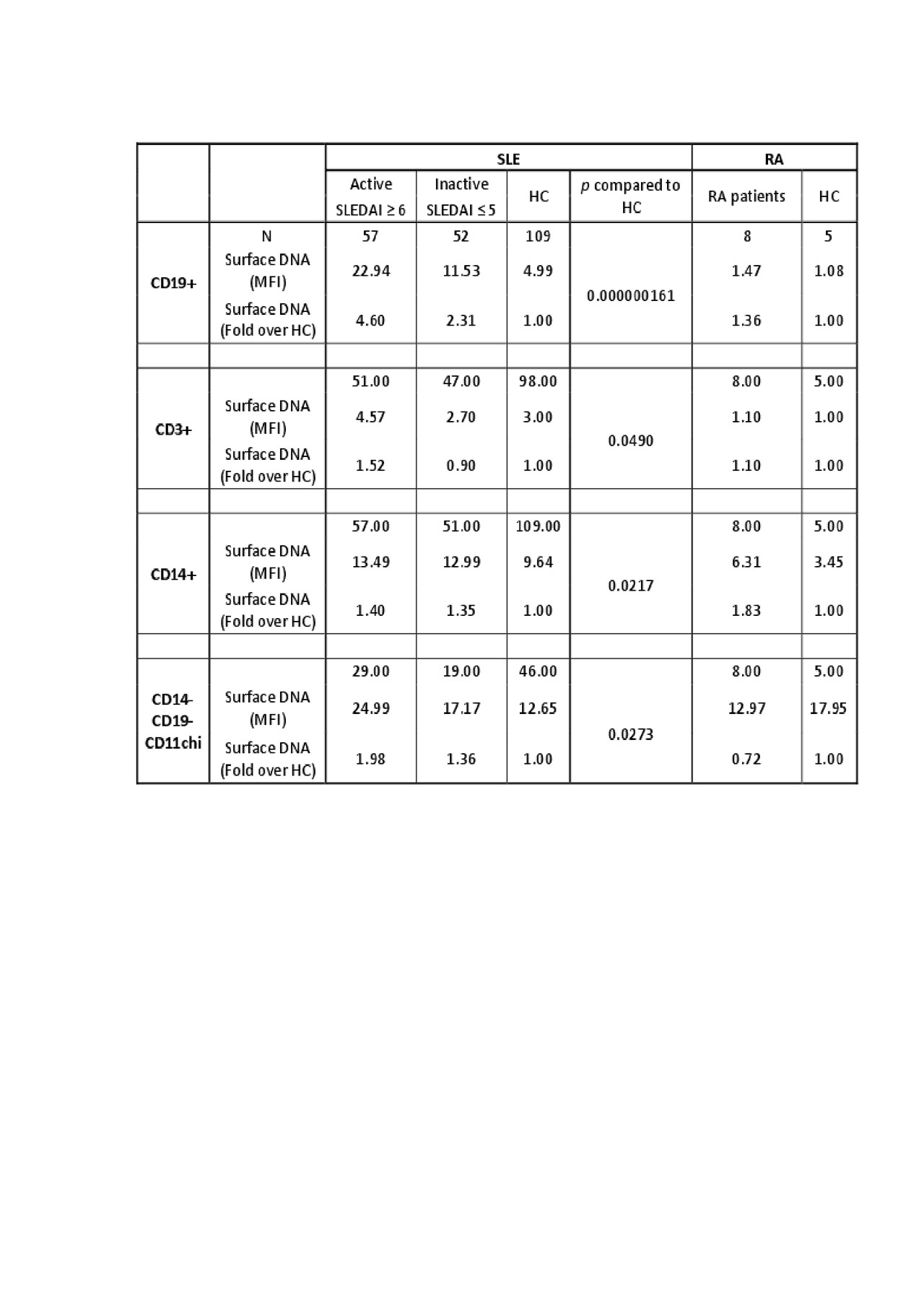
To define whether altered lysosome function promoted accumulation of IgG-ICs, we measured surface DNA levels in 57 active and 51 inactive patients and 109 HCs. We found a significant increase in surface DNA on B cells (4.6 fold) and dendritic cells (2.0 fold), but not on T cells (1.5 fold) or monocytes (1.4 fold) in active SLE vs. HC. Levels of surface DNA on B cells were significantly higher in active versus inactive patients (Table 2). Conversely, 14 active RA patients did not show elevated surface DNA or CCP on any cell type.
Conclusion: We show that monocytes and B cells from active, but not inactive, SLE patients exhibit significantly diminished lysosome function, and that B cells and DCs accumulate surface DNA. The lower level of antigen on monocytes may reflect migration of activated monocytes from blood, because in mice, we also observed that blood monocytes do not exhibit high levels of surface nuclear antigens unlike splenic macrophages from the same animal. The mechanisms underlying accumulation of nuclear antigen on T cells remain unclear. Last, elevated levels of nuclear antigens were not present on active RA controls, suggesting that the defect is driven by mechanisms unique to lupus.
To cite this abstract in AMA style:
Kang S, Rogers J, Sheikh S, Clowse M, Criscione-Schreiber L, Vilen B. Lysosome Defects in SLE Promote the Accumulation of Nuclear Antigens on the Surface of Hematopoietic Cells [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/lysosome-defects-in-sle-promote-the-accumulation-of-nuclear-antigens-on-the-surface-of-hematopoietic-cells/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/lysosome-defects-in-sle-promote-the-accumulation-of-nuclear-antigens-on-the-surface-of-hematopoietic-cells/