Session Information
Date: Sunday, November 12, 2023
Title: Abstracts: SLE – Diagnosis, Manifestations, & Outcomes I: Biomarkers
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: Systemic lupus erythematosus (SLE) is associated with gut microbiota dysbiosis, and community imbalance can coincide with flares of Lupus nephritis (LN). Herein, we sought to understand the blood cell gene expression patterns and antibody responses to a gut pathobiont in longitudinal assessments of SLE patients with LN and concurrent gut colonization blooms of Blautia (Ruminococcus) gnavus (RG).
Methods: In a well-characterized SLE cohort, we performed bulk RNA-seq from whole-blood PAXGene tubes. Our cohort consisted of 24 female subjects (8 controls, 8 with non-renal SLE, 4 LN without RG blooms, and 4 LN concurrent with RG blooms) with longitudinal assessment of whole-blood RNA sequencing, lupus activity, and serum anti-RG lipoglycan antibody levels. After quality control and filtering, gene expression profiles were analyzed. Serum levels of the platelet factors, P-selectin and platelet factor 4 (PF4), were assessed by ELISA.
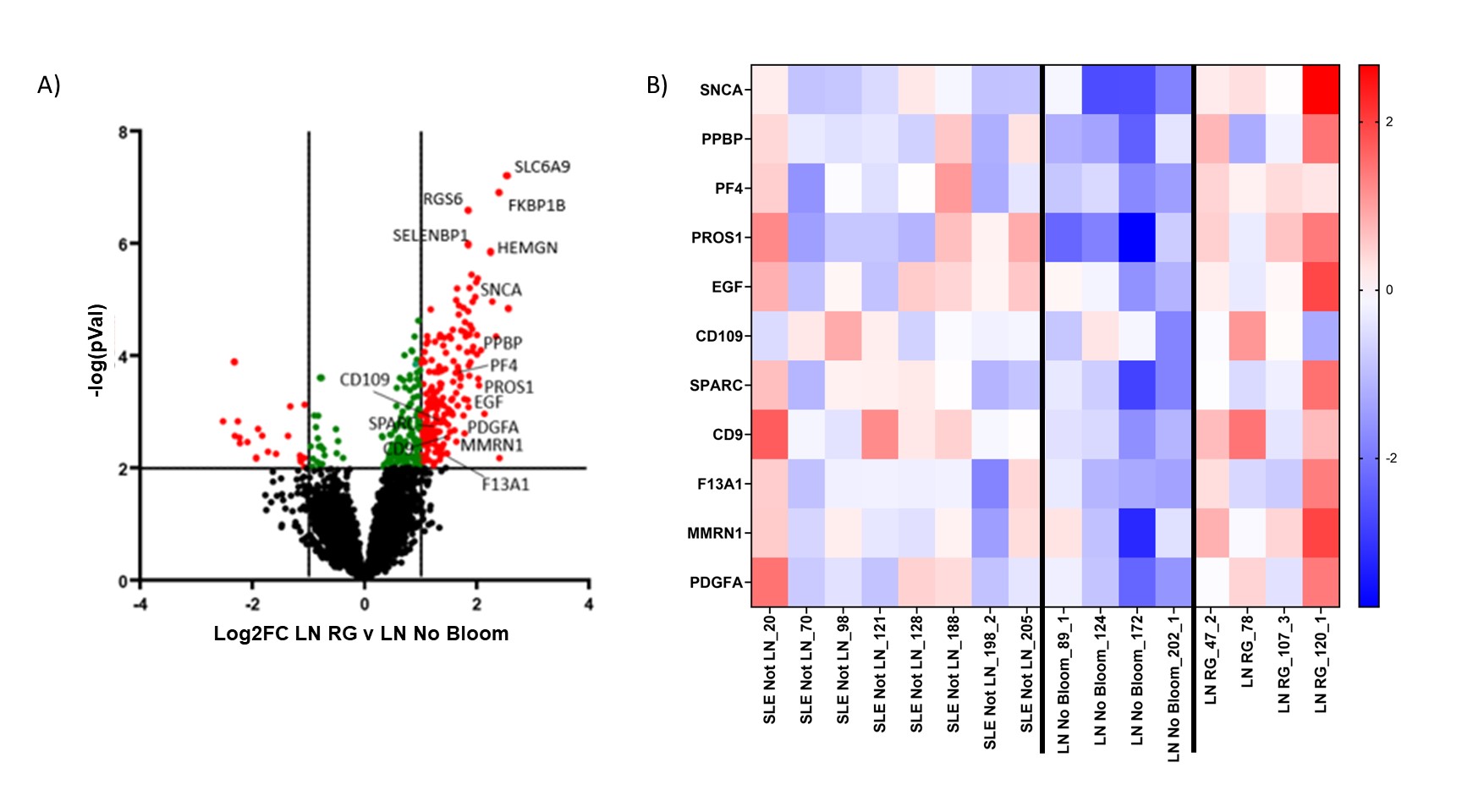
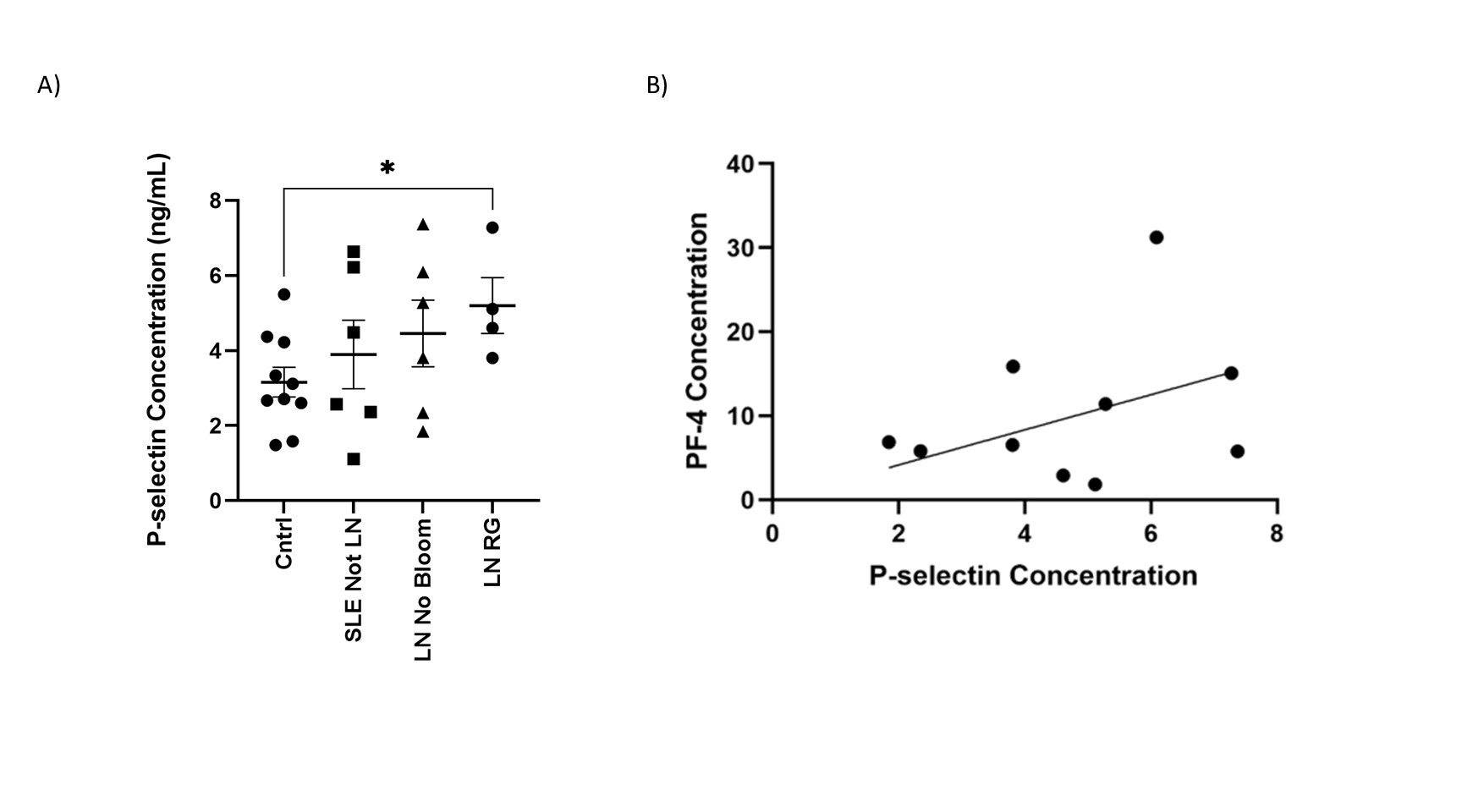
Results: Differential expression and unsupervised clustering analysis revealed distinct gene expression patterns between LN patient without and with RG gut blooms (372 differentially expressed genes; p< 0.01. Gene set enrichment analysis of the differentially expressed genes revealed significant enrichment for “platelet activation”, “platelet alpha granule” and “platelet degranulation” pathways (Figure 1A, FDR < 0.001). Gene expression levels in SLE samples were compared to controls (Figure 1B). As platelet degranulation was found to be upregulated in a subset of LN patients with RG blooms, we interrogated serum levels of P-selectin/CD62P, a protein produced by activated platelets affecting leukocyte activation/migration. We found that P-selectin was increased in LN patients with RG blooms. No significant difference was observed in LN patient without RG blooms or non-renal SLE patients (Figure 2A). The gene expression of PF4, a chemokine protein primary secreted by platelets upon activation was found to be higher in LN RG, compared to controls (Figure 1A). Serum P-selectin directly correlated with PF4 levels (p=0.003)(Figure 2B).
Conclusion: Patients with LN and RG gut blooms displayed upregulated expression of genes for platelet activation and degranulation. Gut blooms with the pathobiont RG, known to cause gut leakiness from the gut and induce high serum anti-RG lipoglycan antibodies, are herein associated with increased platelet activation and raised serum P-selectin and PF4. Taken together, our data point to a potential role for platelet activation and thrombo-inflammation in LN pathogenesis.
To cite this abstract in AMA style:
Amarnani A, Azzouz D, Cornwell M, Mieles D, Izmirly P, Buyon J, Ruggles K, Silverman G. Lupus Nephritis Flares Linked to Platelet Activation During Gut Pathobiont Blooms [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/lupus-nephritis-flares-linked-to-platelet-activation-during-gut-pathobiont-blooms/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/lupus-nephritis-flares-linked-to-platelet-activation-during-gut-pathobiont-blooms/