Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: The Latin American Group for the Study of Lupus (GLADEL) 2.0 is an observational prevalent and incident cohort of patients with systemic lupus erythematosus (SLE) in Latin-America countries. Here we described the rate of treatment response at 12 months in a cohort of SLE patients with active lupus nephritis (LN).
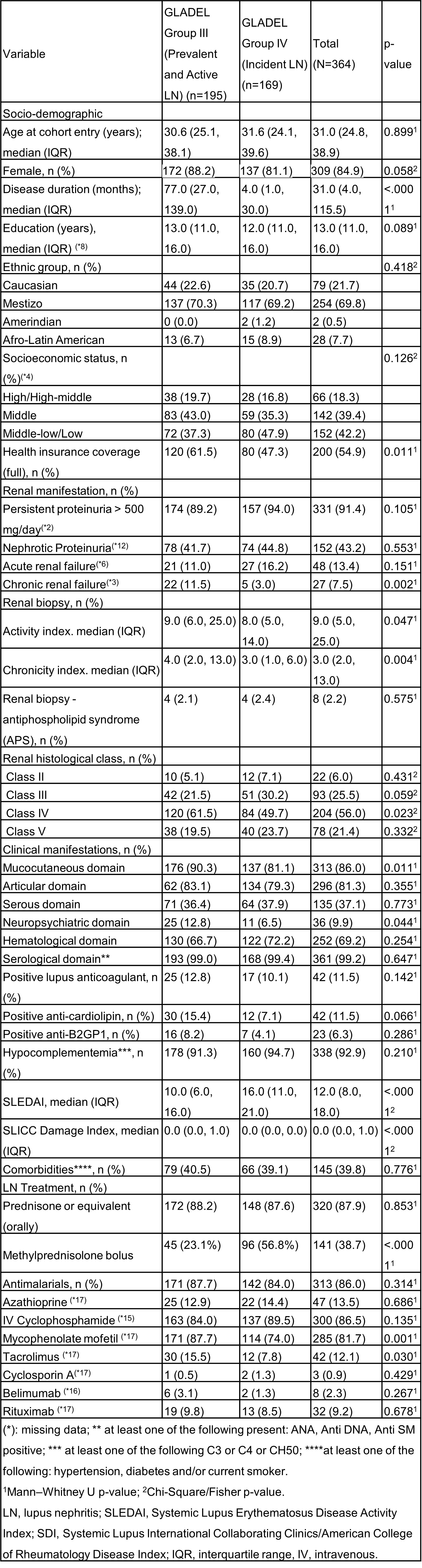
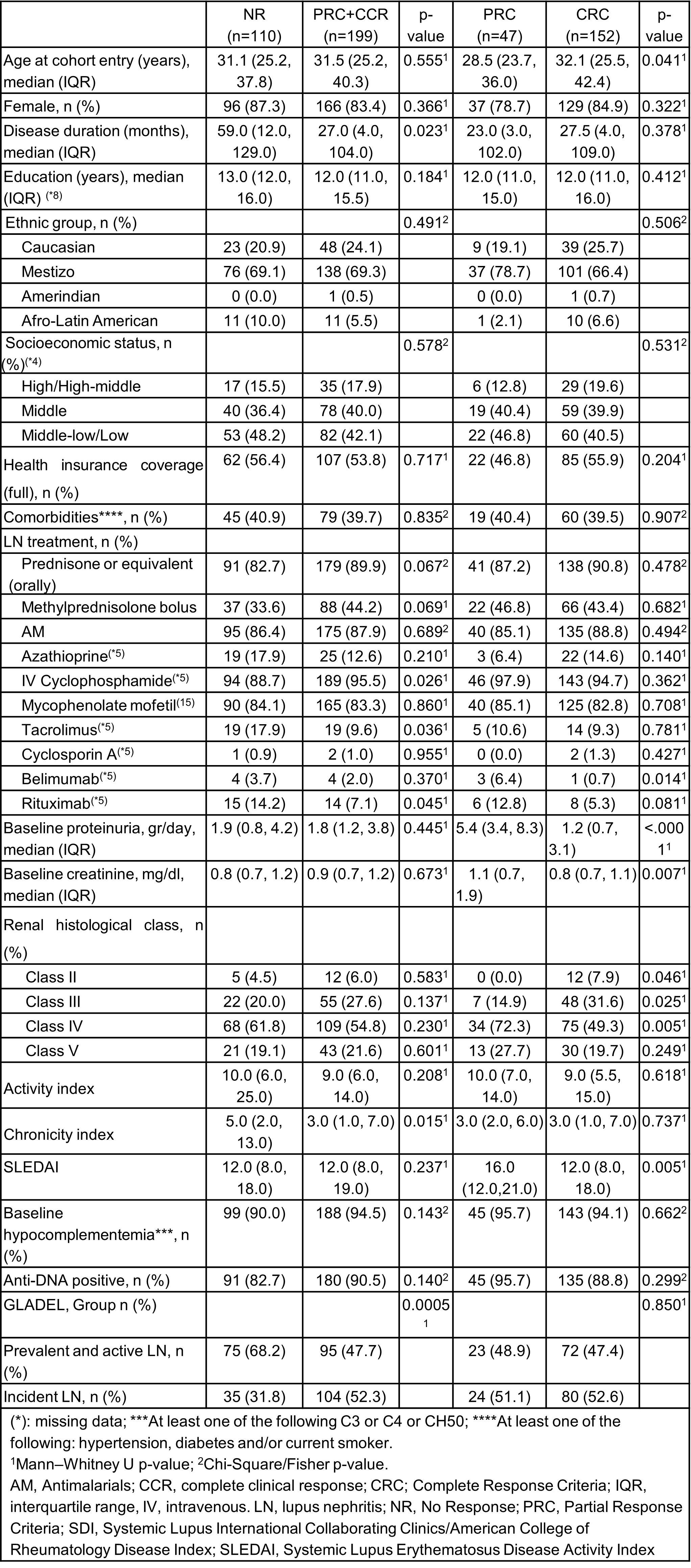
Methods: Forty-four centers from 10 Latin-American countries enrolled patients ≥18 years old who fulfilled the 1982/1997 American College of Rheumatology (ACR) and/or 2012 Systemic Lupus International Collaborating Clinics (SLICC) classification criteria. Patients were categorized into 4 subsets according to the severity of LN. For this analysis, patients in Group III (prevalent and active LN) and IV (incident LN, onset < 3 months with renal biopsy) and sufficient follow-up data at 12 months were included. Baseline demographics, clinical manifestations, disease activity (SLEDAI-2k) and SLICC/ACR Damage Index (SDI) and LN treatments were examined. Partial and complete response according to EULAR/Kidney Disease Improving Global Outcomes (KDIGO) were examined at 12 months: Complete Response Criteria (CRC): proteinuria < 0.5 g/g measured as the urine protein to creatinine ratio (UPCR) from a 24-hour urine collection; Partial Response Criteria (PRC): ≥50% reduction in UPCR from a 24-hour urine collection; and No Response (NR): < 50% reduction in proteinuria.
Results: One-thousand eighty-one patients were enrolled in GLADEL 2.0 with 364 patients included in this analysis: 195 (53.5%) in Group III and 169 (46.4%) in Group IV. At the 12-month follow-up, 13/364 (3.5%) patients had died, 14/364 (3.8%) had been lost to follow-up, and 28/364 (7.6%) had incomplete data; therefore, the calculation of renal response was carried out in the remaining 309 patients. Table 1 describes the characteristics of patients with LN. Table 2 shows that patients who achieved renal response (complete or partial) had a shorter disease duration, greater use of pulse corticosteroids and IV cyclophosphamide, a lower chronicity index and all belonged to the LN incident group. When comparing complete vs partial response, patients who achieved complete response had lower baseline proteinuria and creatinine values, belonged to histological Class III and had lower SLEDAI.
Conclusion: Renal response was achieved in 64% of patients having their first episode of LN, with lower chronicity rates in the biopsy and a lower SLEDAI. Pulsed corticosteroids and IV cyclophosphamide continue to be the options chosen by treating physicians. More data in the follow-up will allow us to evaluate the persistence of this response over time and what factors may influence it.
To cite this abstract in AMA style:
Quintana R, Nieto R, Ávila D, Serrano Morales R, Harvey G, Hernandez L, Roberts K, Scolnik M, Funes Soaje C, Alba P, Saurit V, Garcia M, Berbotto G, BELLOMIO V, Grageda W, Gómez G, Pisoni C, Malvar A, Juarez V, Da Silva N, MONTICIELO O, Mariz H, Ribeiro F, Borba E, Parente L, Torres E, Neira O, Massardo L, Aroca Martínez G, Davila C, López G, Toro-Gutierrez C, Moreno M, Zuñiga A, Saavedra Salinas M, Hernandez M, Fragoso-Loyo H, Silveira Torre L, De La Torre I, Mendoza C, Hernández M, Esquivel-Valerio J, Colman I, Losanto J, Mora Trujillo C, Corrales K, Louis R, Rebella M, Danza Á, Ugarte-Gil M, Alarcon G, Sbarigia U, Zazzetti F, Orillion A, Pons-Estel G, Pons-Estel B. Lupus Nephritis and Response to Treatment in Latin America [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/lupus-nephritis-and-response-to-treatment-in-latin-america/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/lupus-nephritis-and-response-to-treatment-in-latin-america/