Session Information
Date: Friday, November 6, 2020
Title: SLE – Diagnosis, Manifestations, & Outcomes Poster I: Clinical Manifestations
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The Accelerating Medicines Partnership (AMP) will use multi-omics modalities including single cell RNA sequencing to understand lupus nephritis with the ultimate goal to devise novel and personalized treatment strategies. African-Americans have more lupus nephritis and worse outcomes in terms of end stage renal disease. We report here the clinical findings to date on African-American patients in the AMP cohort.
Methods: We included 118 patients with urine protein-to-creatinine ratio (UPCR) ≥ 1 and biopsy proven class III, IV, V or mixed lupus nephritis at time of enrollment. All patients met revised ACR or SLICC classification criteria. Clinical data were obtained at baseline, 12, 26, and 52 weeks after the renal biopsy. Response status at week 52 was defined as follows. Complete: UPCR ≤ 0.5, normal serum creatinine (sCr) or < 25% increase from baseline if abnormal, and prednisone < 10mg daily; partial: UPCR > 0.5 but ≤ 50% of the baseline value and same sCr and prednisone rules as complete response; no response: UPCR > 50% of baseline value or new abnormal elevation of sCr or ≥ 25% from baseline or prednisone ≥ 10mg daily.
Results: Table 1 shows that African Americans were more likely to have class V lupus nephritis (38% vs 22.5%, p=0.06), were less serologically active (low C3 50% vs 77.5%, p=0.002; anti-dsDNA 63% vs 79%, p=0.006), and were more likely to have elevated serum creatinine (55% vs 30%, p=0.03). Caucasians were older (47 vs 34 years, p=< 0.001) and more likely to be at their first biopsy (64% vs 31%, p=0.04). Table 2 shows the differences based on the first biopsy versus a repeat biopsy. African-Americans were significantly less likely to have a treatment response at the first biopsy. Regardless of first or later biopsy, they were less likely to have low C3. Table 3 shows multi-variate models. African-American patients at their first episode of lupus nephritis were less likely to respond to treatment (37.5% vs 75%, p=0.018) independently of histological features including class, activity and chronicity.
Conclusion: The AMP cohort demonstrates the current unmet clinical need to improve treatment of lupus nephritis in the United States. African-American lupus nephritis is different in terms of ISN class, serologies, first biopsy, and worse in terms of response status even after adjusting for activity and chronicity. Personalized treatments should be developed to improve outcomes in high risk populations such as African-Americans.
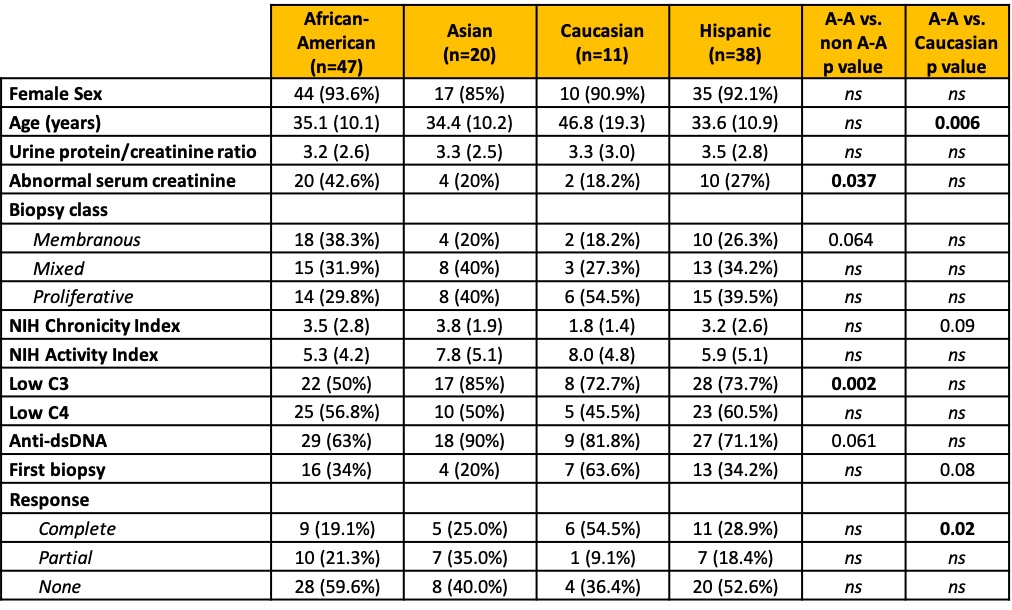 Table 1. Patients characteristics by race / ethnicity. Data are presented as n (%) or mean (SD). Two patients identified as “Other” and are not shown in this table. P values > 0.1 are indicated as ns.
Table 1. Patients characteristics by race / ethnicity. Data are presented as n (%) or mean (SD). Two patients identified as “Other” and are not shown in this table. P values > 0.1 are indicated as ns.
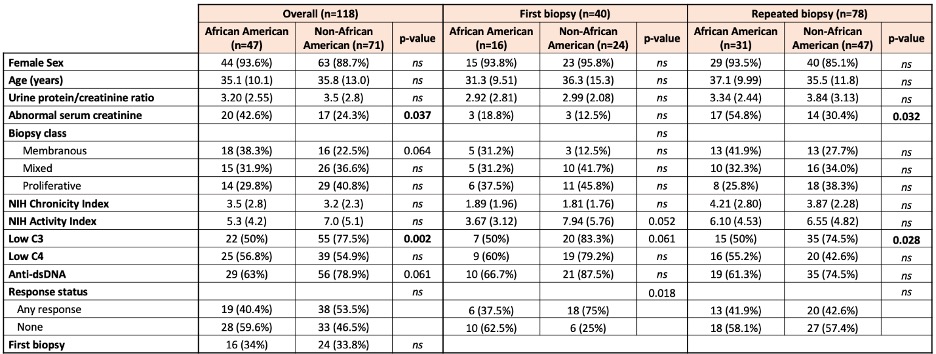 Table 2. Comparison of clinicodemographic features between African-American and non African-American patients at first or repeat biopsy. Data are presented as n (%) or mean (SD). P values > 0.1 are indicated as ns.
Table 2. Comparison of clinicodemographic features between African-American and non African-American patients at first or repeat biopsy. Data are presented as n (%) or mean (SD). P values > 0.1 are indicated as ns.
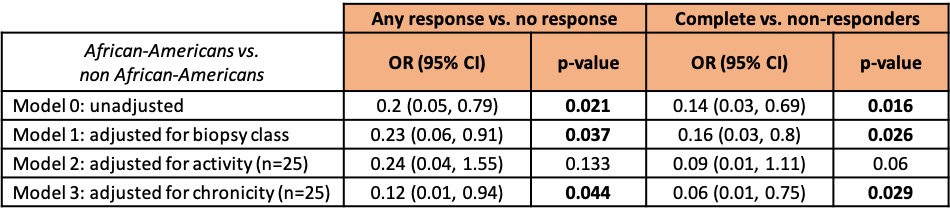 Table 3. Multivariable models of response status in African-American and non African-American patients. Only patients at their first biopsy were included.
Table 3. Multivariable models of response status in African-American and non African-American patients. Only patients at their first biopsy were included.
To cite this abstract in AMA style:
Fava A, Li J, Carlucci P, Wofsy D, James J, Putterman C, Diamond B, Fine D, Monroy-Trujillo J, Haag K, Deonaraine K, Accelerating Medicines Partnership in SLE Network T, Apruzzese W, Buyon J, Petri M. Lupus Nephritis and Renal Outcomes in African-Americans: The Accelerating Medicines Partnership Cohort Experience [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/lupus-nephritis-and-renal-outcomes-in-african-americans-the-accelerating-medicines-partnership-cohort-experience/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/lupus-nephritis-and-renal-outcomes-in-african-americans-the-accelerating-medicines-partnership-cohort-experience/
