Session Information
Date: Sunday, October 21, 2018
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Secukinumab, a fully human monoclonal antibody that neutralizes IL-17A, improved signs and symptoms of ankylosing spondylitis (AS) in patients (pts) in the MEASURE 1 core trial at 2 years and through 4 years in the extension study.1,2 A low radiographic progression rate was also reported through 2 years (Δ mSASSS at Year 2=0.3).1 Comparison of anti-TNF agents with historical NSAID-treated cohorts have not shown a significant benefit at 2 years in reducing radiographic progression.3,4 This retrospective analysis compared spinal radiographic progression over 2 years in the MEASURE 1 cohort of secukinumab-treated AS pts (C1; NCT01358175) vs a historical cohort of biologic-naïve AS pts (ENRADAS [C2; NCT00715091]).5
Methods: Baseline (BL) and 2-year X-ray data from the 2 cohorts were compared. Only data from pts with X-rays at BL (up to Day 30) and Year 2 (Days 31–743) were included (n=168 [C1], n=69 [C2]). X-rays were independently re-evaluated using the mSASSS by 2 reviewers (and an adjudicator for the top 10% of cases with the highest difference in Δ mSASSS between readers) who were blinded to sequence and treatment; averaged values (or the adjudicated score) were analysed. The primary outcome was to compare the % pts with no radiographic progression (Δ mSASSS at Year 2 ≤0) in C1 vs C2. The difference between C1 and C2 was analysed using a logistic regression with cohort as a factor and BL mSASSS as a covariate. Sensitivity analyses were performed to assess the robustness of the comparison between the two cohorts using sensitivity analysis set 1 (BL to Days 640–819) and sensitivity analysis set 2 (all subjects with BL and post-BL X-rays [time-adjusted and unadjusted]).
Results: BL demographics were comparable across cohorts, with mean age 40.9 vs 42.6 years, and gender 72.8% vs 66.7% male in C1 vs C2, respectively. Over 2 years, least squares (LS) mean Δ mSASSS was 0.55 for C1 vs 0.89 for C2 (p=0.185) and % pts with no radiographic progression (Δ mSASSS at Year 2 ≤0) was slightly higher in C1 vs C2. Mean changes from BL in mSASSS were numerically lower in the MEASURE 1 vs ENRADAS cohorts across all the analyses performed (Table).
Conclusion: Over 2 years, a numerically lower rate of progression was seen in secukinumab-treated pts vs a control cohort of biologic-naïve AS pts. Further research is needed to understand the impact of IL-17A inhibition with secukinumab on spinal disease progression in AS pts; SURPASS (NCT03259074), an ongoing H2H study powered to compare differences in spinal radiographic progression with secukinumab vs biosimilar adalimumab, will help answer these questions. References: 1. Braun J et al. Ann Rheum Dis Ann Rheum Dis 2017;76:1070–77; 2. Braun J et al. Arthritis Rheumatol 2017;69(10). 3. van der Heijde D et al. Arthritis Rheum 2008;58:1324–31; 4. van der Heijde D et al. Arthritis Res Ther 2009;11:R127; 5. Sieper J et al. Ann Rheum Dis 2016;75:1438–43.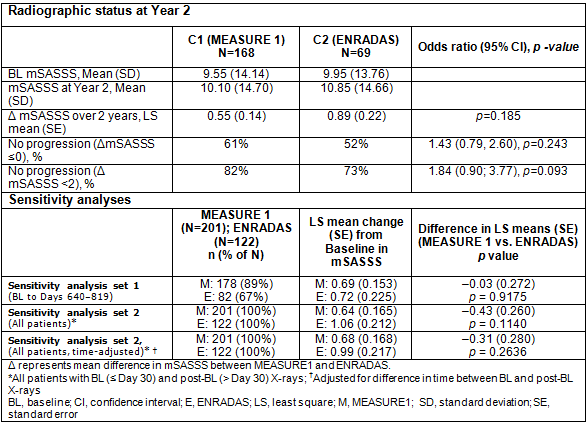
To cite this abstract in AMA style:
Braun J, Haibel H, de Hooge M, Landewé RBM, Rudwaleit M, Fox T, Readie A, Richards H, Porter B, Martin R, Poddubnyy D, Sieper J, van der Heijde D. Low Rate of Spinal Radiographic Progression over 2 Years in Ankylosing Spondylitis Patients Treated with Secukinumab: A Historical Cohort Comparison [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/low-rate-of-spinal-radiographic-progression-over-2-years-in-ankylosing-spondylitis-patients-treated-with-secukinumab-a-historical-cohort-comparison/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/low-rate-of-spinal-radiographic-progression-over-2-years-in-ankylosing-spondylitis-patients-treated-with-secukinumab-a-historical-cohort-comparison/
