Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Guidelines recommend adjusting therapy in patients with RA who fail to reach low disease activity or remission. Partial responders to a TNF inhibitor may decline treatment changes for fear of clinical worsening and losing the response they had so far achieved. Little is known about the likelihood of worsening after switching therapy to a drug with a different mechanism of action, and this limits patients’ ability to make informed decisions. We performed a post hoc analysis to assess the effect of switching from adalimumab to sarilumab in patients with RA who partially responded to adalimumab in MONARCH (NCT02332590), a head-to-head study of sarilumab versus adalimumab monotherapy. At the end of the trial, patients on adalimumab were switched to sarilumab 200 mg q2w for the open-label extension (OLE).
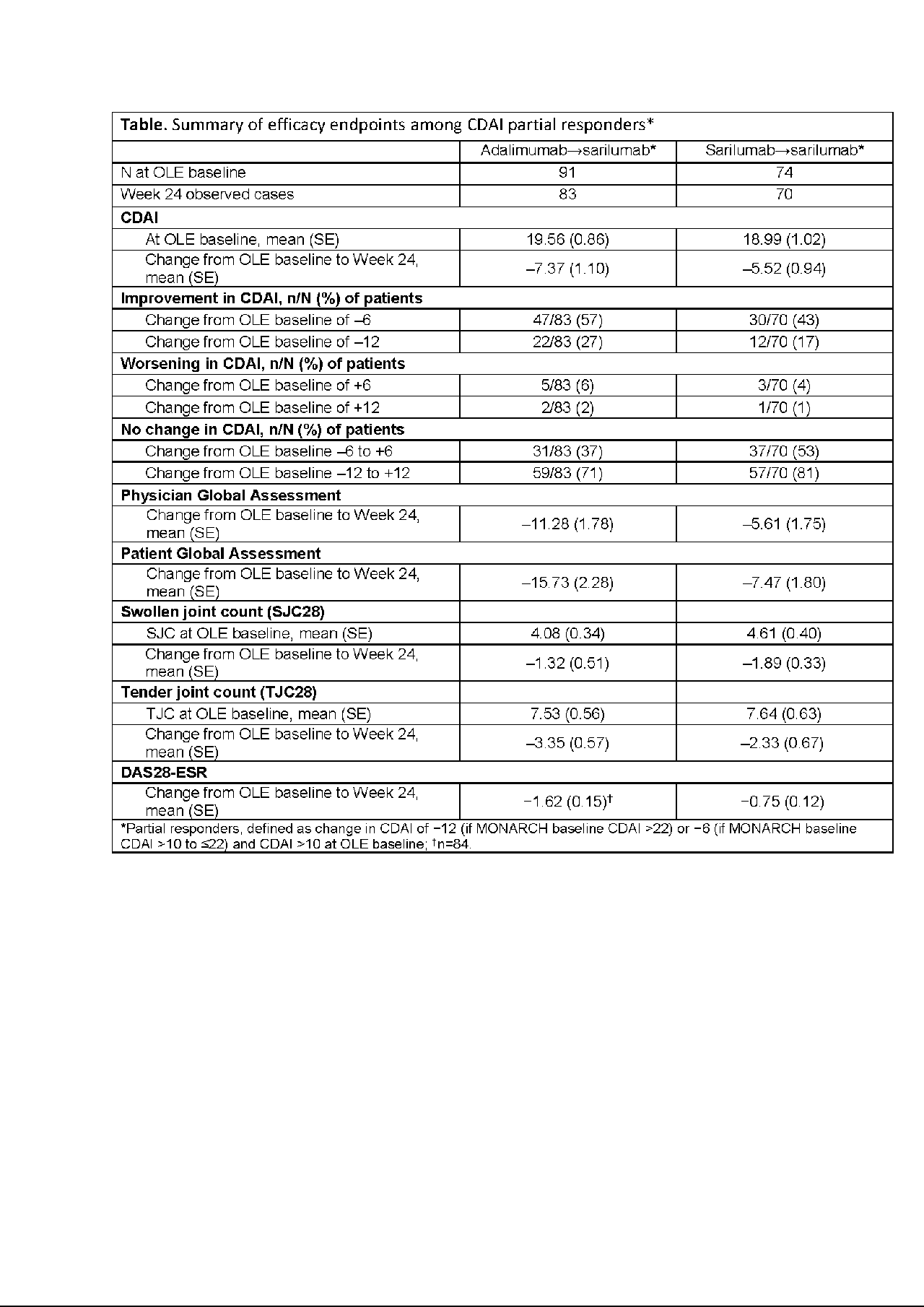
Methods: Thresholds of change in Clinical Disease Activity Index (CDAI) of ±6 and ±12 units were used to define meaningful improvement/worsening, anchored at the OLE baseline (BL). Partial responders to adalimumab or sarilumab were characterized at OLE BL and defined as having had a clinically meaningful change in CDAI (≤−12 if MONARCH BL CDAI >22 or ≤−6 if MONARCH BL CDAI >10 to ≤22) during MONARCH while still having moderate-to-high disease activity (CDAI >10) at OLE BL. Response was assessed by change in CDAI from OLE BL at Week 24. The proportions of partial responders achieving meaningful improvement or worsening were calculated.
Results: There were 369 patients enrolled in MONARCH, of whom 320 (87%) entered OLE—155 switching from adalimumab to sarilumab, 165 continuing sarilumab. Of the patients in OLE, 52% were partial responders at OLE BL: switch, n=91/155 (59%); continuation, n=74/165 (45%). Mean (SE) CDAI at OLE BL was 19.56 (0.86) in switch partial responders and 18.99 (1.02) in continuation partial responders, with mean (SE) change in CDAI from OLE BL at Week 24 of −7.37 (1.10) and −5.52 (0.94), respectively (Table). Based on a CDAI threshold of ±6, improvement in disease activity was observed in 57% (switch) and 43% (continuation), while worsening was observed in 6% and 4% of patients. No change (−6 to +6) was evident in 37% (switch) and 53% (continuation). Based on a CDAI threshold of ±12, improvement in disease activity was observed in 27% (switch) and 17% (continuation), while worsening was observed in 2% and 1%, and no change (−12 to +12) was evident in 71% and 81%, respectively. Other efficacy measures demonstrated improvements from baseline (Table). No new safety signals emerged during OLE, and the adverse event profile among switch patients was similar to that of continuation patients.
Conclusion: Among adalimumab partial responders who switched to sarilumab, there was a low (6%) likelihood of clinical worsening, and almost all patients (94%) experienced clinical improvement or had no change. These findings can help inform patients’ and clinicians’ shared decision making when considering changes in RA therapy.
To cite this abstract in AMA style:
Curtis J, Aletaha D, Burmester G, Ford K, van Hoogstraten H, Leher H, Thangavelu K, Bykerk V. Low Probability of Clinical Worsening Following Switching Biologic DMARD in Patients with RA and Partial Response to Adalimumab [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/low-probability-of-clinical-worsening-following-switching-biologic-dmard-in-patients-with-ra-and-partial-response-to-adalimumab/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/low-probability-of-clinical-worsening-following-switching-biologic-dmard-in-patients-with-ra-and-partial-response-to-adalimumab/