Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: By converting extracellular ATP into homeostatic adenosine, the ectonucleotidases CD39 (ATP to AMP) and CD73 (AMP to adenosine) dictate extracellular purinergic gradients and as such are critical players in the regulation of leukocyte and platelet functions. Insufficient activity of these homeostatic ectoenzymes has been associated with neutrophil-driven inflammation. Crosstalk between neutrophils and platelets links thrombotic and inflammatory processes, and elevated levels of neutrophil-platelet aggregates (NPAs) have been reported in various disease states. Understudied are (i) the role that ectonucleotidases play in restraining NPA formation, as well as (ii) the role that NPAs play in the prototypical thromboinflammatory disease, antiphospholipid syndrome (APS). Here, we aimed to study the relationship between the CD39/CD73 axis, neutrophils, and platelets in APS pathophysiology.
Methods: NPAs (CD16+CD15+CD41+) and platelet P-selectin (CD62P) levels were assessed in the blood of patients with durably positive antiphospholipid antibodies (aPL) and features of APS (n=36) by flow cytometry; no subjects had lupus. These 36 subjects were compared to 20 healthy controls. In parallel, neutrophil CD39 and CD73 activities were measured by a standard malachite green assay. Healthy donor neutrophils and platelets (ratio 1:20) were also studied in vitro in the presence or absence of a specific CD39 activity inhibitor (ARL 67156). Potential blockers of NPA formation were also tested including anti-CD62P and anti-CD162 (anti-PSGL-1).
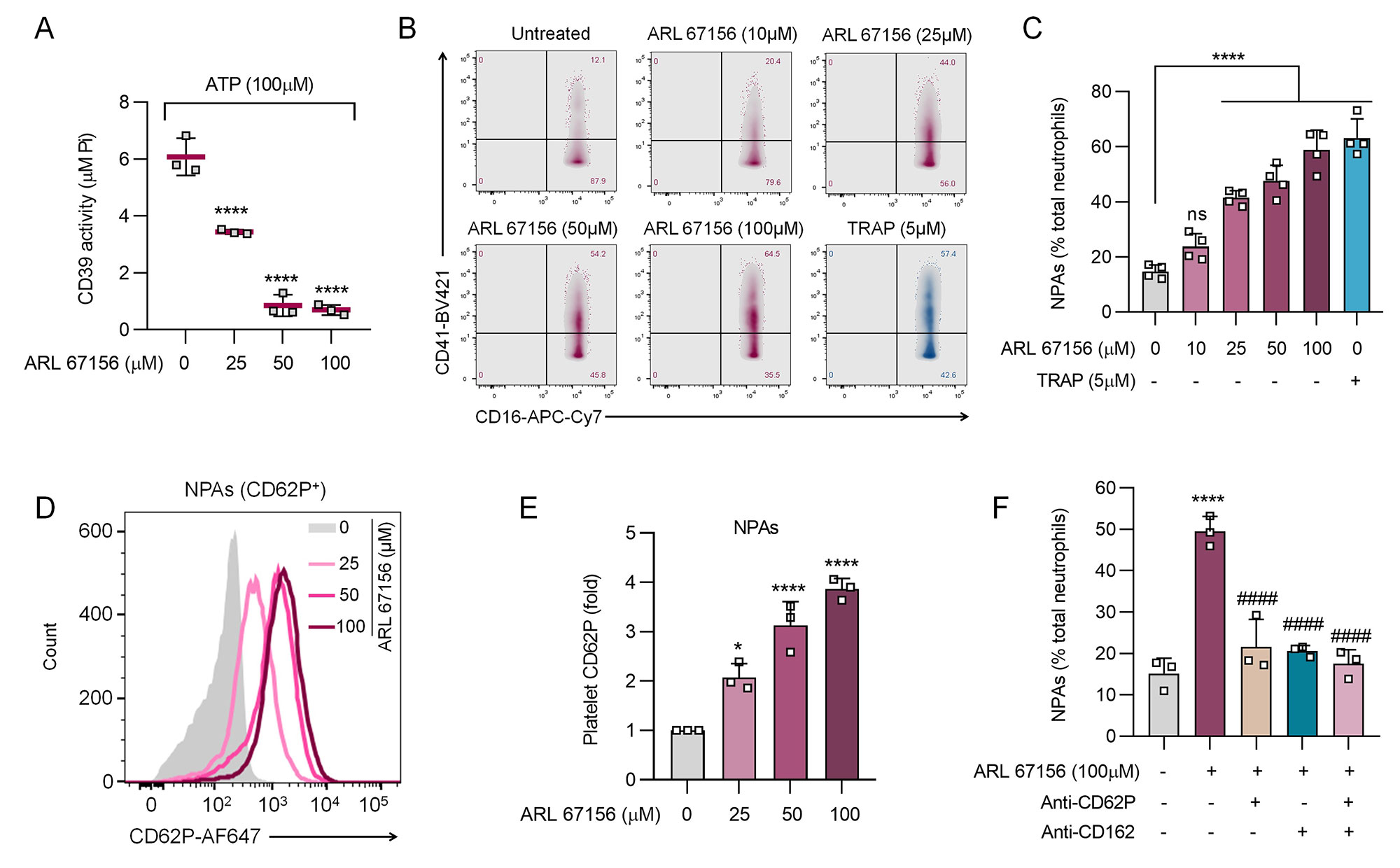
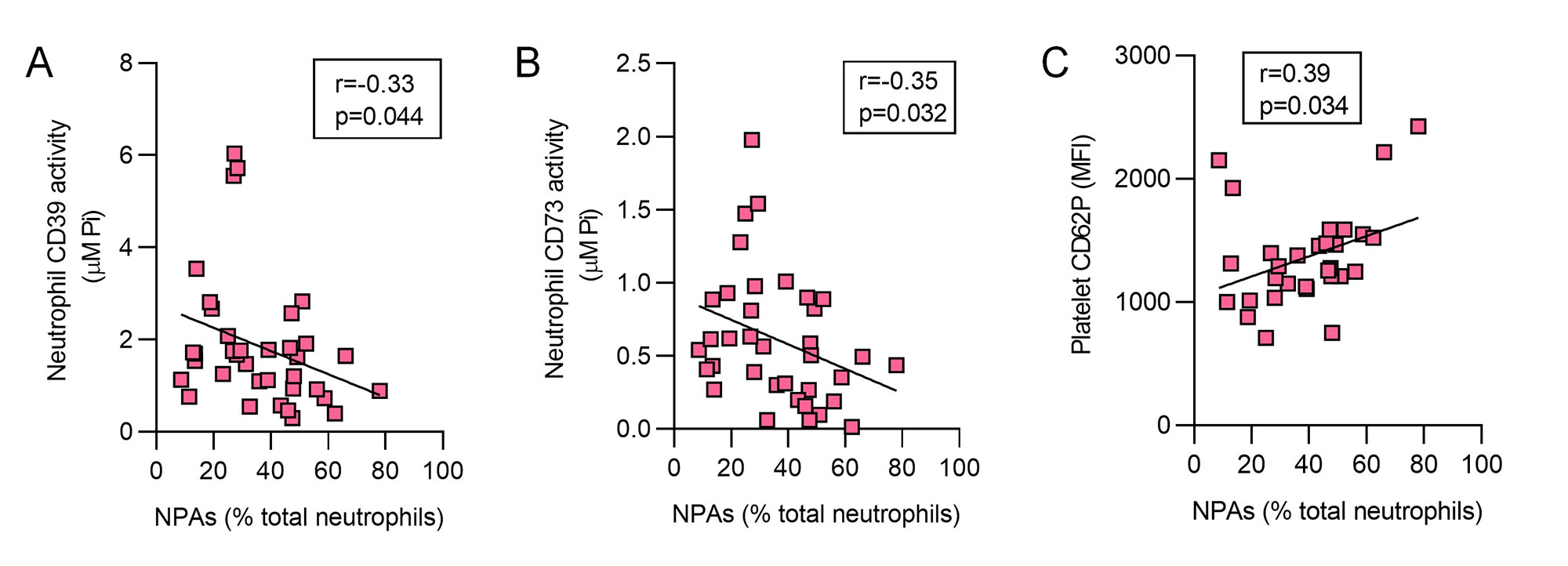
Results: In 36 aPL-positive patients, activities of both neutrophil CD39 (p< 0.001) and neutrophil CD73 (p< 0.001) were markedly decreased as compared with healthy controls (Fig 1A-B). This was paralleled by significant elevations of NPAs (up to 80% of total neutrophils, p< 0.0001) and platelet activation as defined by CD62P expression (p< 0.05, Fig 1C-D). When healthy neutrophils and platelets were cultured together, inhibition of CD39 activity significantly enhanced NPA formation (from baseline 20% to 70%) in dose-dependent fashion (Fig 2A-C). In the same system, increased platelet activation was also observed (2- to 4-fold), as was inhibition of NPA formation by either anti-CD62P or anti-CD162 (both p< 0.0001, Fig 2D-F). In a correlation analysis focused on the aPL-positive patient samples (n=36), circulating NPAs were inversely correlated with neutrophil CD39 activity (r=-0.33, p=0.044) and CD73 activity (r=-0.35, p=0.032, Fig 3A-B). Levels of NPAs also tracked with platelet activation (r=0.38, p=0.034, Fig 3C).
Conclusion: We provide evidence that diminished CD39 and CD73 activity (as appears to be present in many patients with APS) potently potentiate the formation of NPAs. In this context, blocking either CD62P or CD162 significantly tempers NPA formation. Taken together, these findings have potential to identify new approaches to therapy and sub-phenotyping for patients on the APS disease spectrum. Experiments are now underway to determine the upstream regulators of ectonucleotidase activity in APS as well as the downstream pathogenic consequences of excessive NPAs formation.
To cite this abstract in AMA style:
S K N, Hoy C, Yalavarthi S, Sarosh C, Sabb K, Rysenga C, Madison J, Zuo Y, Knight J. Low Neutrophil Ectonucleotidase Activity Drives Neutrophil-Platelet Aggregation and Platelet Activation in Antiphospholipid Syndrome (APS) [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/low-neutrophil-ectonucleotidase-activity-drives-neutrophil-platelet-aggregation-and-platelet-activation-in-antiphospholipid-syndrome-aps/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/low-neutrophil-ectonucleotidase-activity-drives-neutrophil-platelet-aggregation-and-platelet-activation-in-antiphospholipid-syndrome-aps/