Session Information
Date: Monday, November 8, 2021
Title: SLE – Diagnosis, Manifestations, & Outcomes Poster III: Outcomes (1257–1303)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: The Accelerating Medicines Partnership (AMP) Lupus Network was established with the goal of applying novel technologies to the interrogation of blood and tissue samples from patients with lupus nephritis (LN). In contrast to global LN clinical trials, the AMP LN cohort affords an opportunity to generate outcome data representative of a US multicenter multi-ethnic real-world experience. In this analysis, the AMP clinical dataset was investigated to determine the percentages of patients who attained prespecified definitions of partial or complete responses at 52 weeks. In addition, incorporation of response rates at weeks 12 and 26 to the analysis provided longitudinal patterns of response to standard of care.
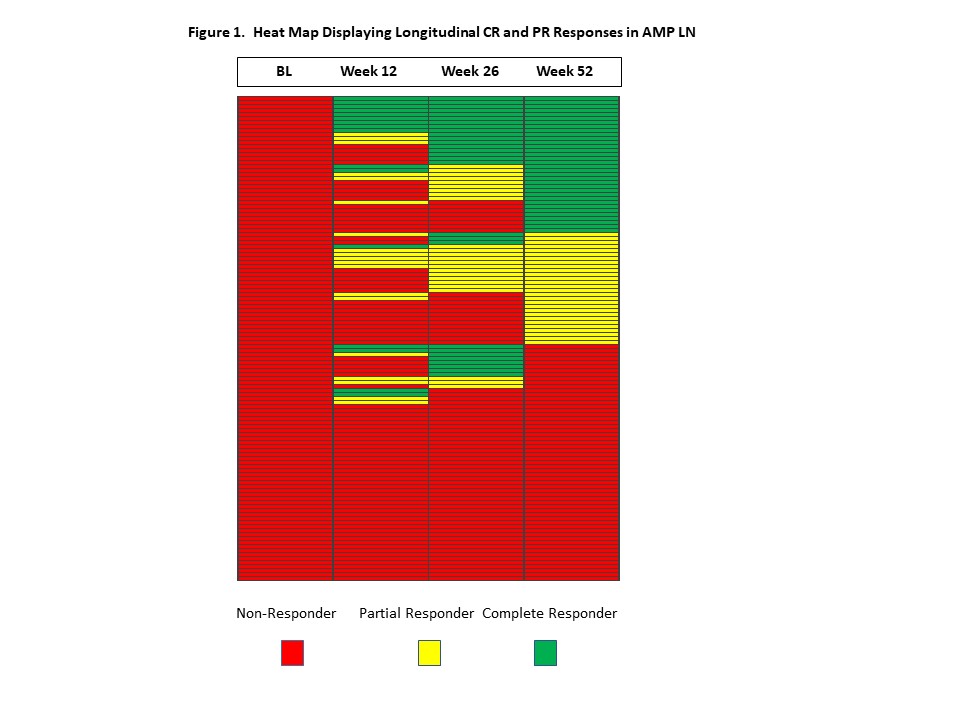
Methods: Patients with LN who were undergoing kidney biopsies as part of standard of care were eligible to enroll in the AMP LN study. Response definitions were only applied to cases whose baseline spot urine protein/creatinine (UPCR) ratios were > 1.0. Complete response (CR) required: 1) UPCR < 0.5; and 2) normal creatinine (< 1.3 mg/dL) or, if abnormal at baseline, < 125% of baseline; and 3) prednisone < 10 mg/day at the time of the study visit. Partial response required: 1) >50% reduction in UPCR without meeting UPCR criterion for CR; and 2) normal creatinine (< 1.3 mg/dL) or, if abnormal, < 125% of baseline; and 3) prednisone dose < 15 mg/day at the time of the study visit. Patients who did not achieve a CR or PR at the specific timepoints were considered non-responders (NR). Only patients with renal biopsies that demonstrated ISN/RPS classes III, IV, V or combined III or IV with V and data available at all four timepoints (baseline, weeks 12, 26 and 52) were included in this analysis. Cross-sectional and longitudinal analyses of responses were performed, and heat maps were generated to graphically display response patterns.
Results: Data on 121 patients with LN enrolled in AMP were included in this analysis. Cross-sectional response rates at 52 weeks were: CR: 28.1%; PR: 23.1%; NR: 48.8% (Table 1). Response rates at weeks 12 and 26 are additionally displayed in Table 1, and Figure 1 is a heat map demonstrating longitudinal responses of our patients. All patients were considered NR at baseline. Only 7.4% of patients had week 12 CR responses sustained through week 52, whereas 19% had attained PR or CR at all 3 visits. An additional 14.9% achieved a PR or CR at 26 weeks which was sustained at 52 weeks. Overall, 36.4% of patients were NR at all time points.
Conclusion: Clinical data from the AMP Lupus Network revealed rates of 52-week CR and PR that were consistent with placebo response data from recently conducted LN trials. Low sustained CR rates not only underscore the need for more efficacious therapies but highlight how critically important it is to understand the molecular pathways that are associated with response and non-response.
To cite this abstract in AMA style:
Izmirly P, Dall'Era M, Kalunian K, Deonaraine K, Kim M, Carlucci P, Li J, Fava A, Belmont H, Putterman C, Anolik J, Diamond B, Wofsy D, Kamen D, James J, (AMP) RA/SLE Network A, Rao D, Accelerating Medicines Partnership in SLE Network T, Petri M, Buyon J, Furie R. Longitudinal Patterns of Response to Standard of Care Therapy for Lupus Nephritis: Data from the Accelerating Medicines Partnership Lupus Network [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/longitudinal-patterns-of-response-to-standard-of-care-therapy-for-lupus-nephritis-data-from-the-accelerating-medicines-partnership-lupus-network/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/longitudinal-patterns-of-response-to-standard-of-care-therapy-for-lupus-nephritis-data-from-the-accelerating-medicines-partnership-lupus-network/