Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
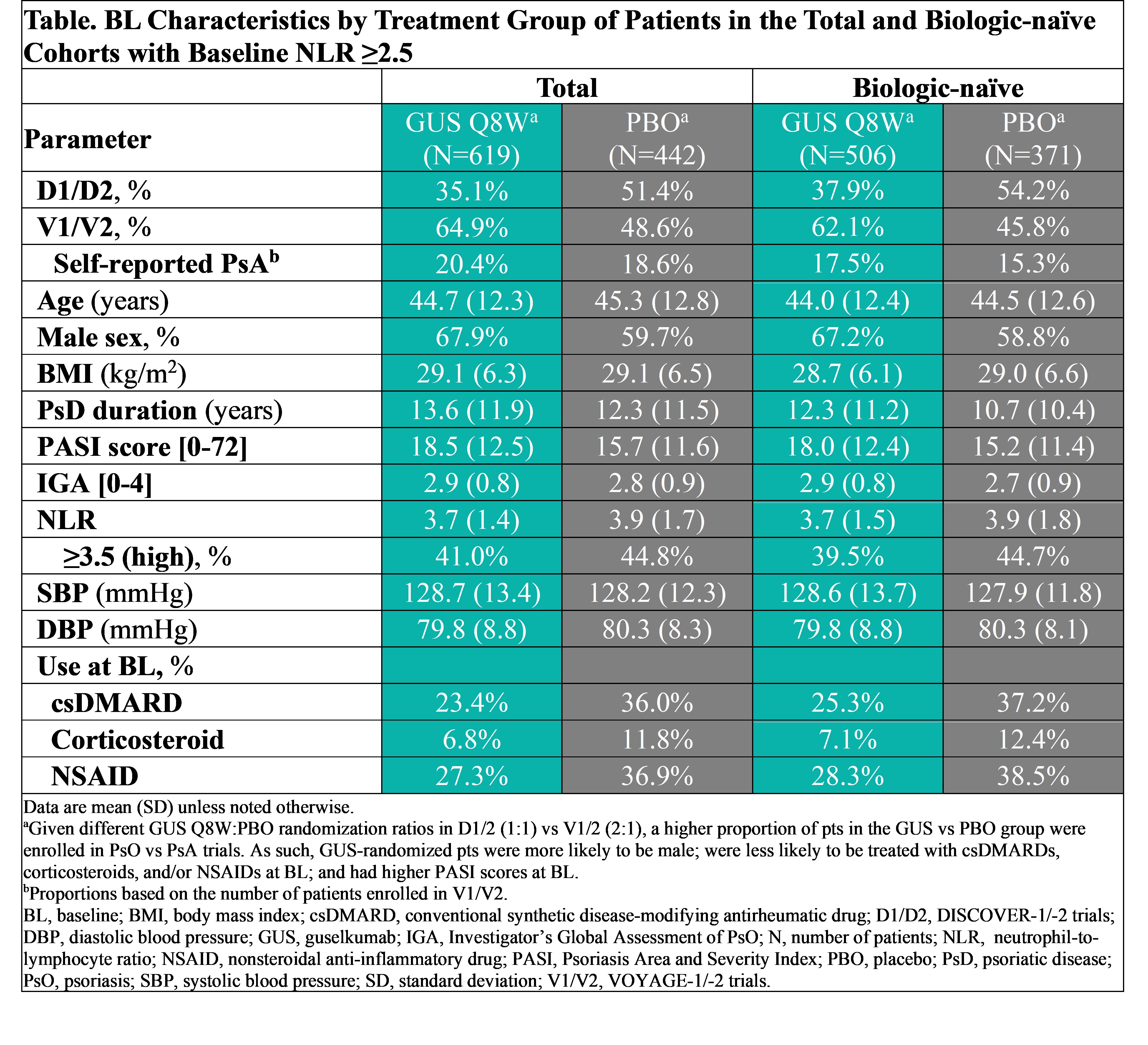
Background/Purpose: Psoriatic disease (PsD) is associated with an increased risk of cardiovascular (CV) disease. Neutrophil-to-lymphocyte ratio (NLR) is a biomarker of systemic inflammation; NLR ≥2.5 to < 3.5 (elevated) or ≥3.5 (high) have shown an independent association with CV risk vs NLR < 2.5.1,2 Guselkumab (GUS) demonstrated significant efficacy in treating multiple PsA domains in the phase 3 DISCOVER (D)1 and 2 studies and psoriasis (PsO) in the phase 3 VOYAGE (V)1 and 2 studies. The effect of GUS on NLR levels in adults with PsD and NLR levels indicative of elevated or high CV risk was evaluated here.
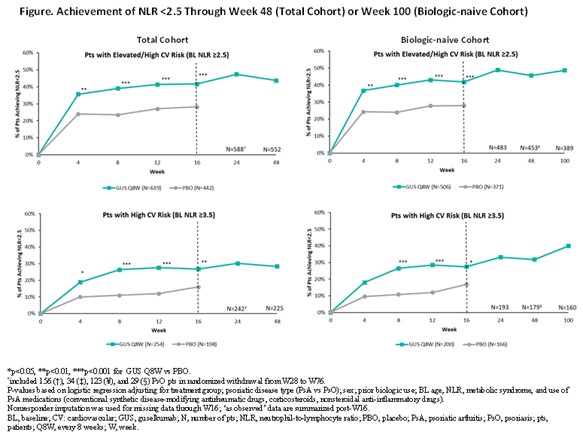
Methods: D1/2 patients (pts) (~90% biologic-naïve) with active PsA were randomized 1:1:1 to GUS 100 mg every 4 weeks (Q4W; N=373); GUS 100 mg at Week (W)0, W4, then Q8W (N=375); or PBO (N=372). V1/2 pts (~80% biologic-naïve) with moderate-to-severe PsO were randomized 2:1 to GUS 100 mg at W0, W4, then Q8W (N=825) or PBO (N=422). GUS Q8W- and PBO-randomized pts with baseline (BL) NLR ≥2.5 were included; subgroup analyses in pts with NLR levels associated with elevated (2.5 to < 3.5) and high (≥3.5) CV risk were performed. Least square mean (LSM) changes in NLR from W0-W100 were assessed with mixed models for repeated measures. In pts with elevated or high CV risk according to BL NLR level, attainment of NLR < 2.5 (associated with no increased risk) through W16 (PBO-controlled period common to all trials) was compared between GUS and PBO with logistic regression (Figure). Changes from W0-W100 in BMI/systolic blood pressure (SBP)/diastolic BP (DBP) were described.
Results: Of 1061 total pts with elevated (57.4%) or high (42.6%) risk based on NLR level, 877 (82.7%) were biologic-naïve. BL characteristics (other than NLR) were comparable between cohorts (Table). After adjusting for potential confounders, and regardless of BL NLR-defined CV risk category, GUS-treated pts exhibited significantly greater reductions in NLR vs PBO at W4 through W16; LSM reductions were sustained through 1 year (Y) (total) and 2Y (biologic-naïve) of GUS (data not shown). In pts with BL elevated/high risk, significantly greater proportions of GUS- vs PBO-randomized pts achieved NLR < 2.5 as of W4 or W8 through W16 (Figure). In GUS-treated biologic-naïve pts with elevated/high CV risk at BL, who were followed through 2Y, rates of achieving NLR level < 2.5 increased from W16 (41.9%/27.5%) to W100 (48.8%/40.0%). Also in pts with elevated/high CV risk, mean BMI/SBP/DBP were stable up to 2Y of GUS (data not shown)
Conclusion: In PsD pts with elevated/high CV risk defined by BL NLR, GUS led to rapid, significant, and sustained reductions in NLR, resulting in substantial proportions of pts exhibiting NLR levels that have in other analyses been associated with no increased CV risk at follow-up. These findings are consistent with the GUS safety profile established through 5Y.
< 1. Adamstein NH. Eur Heart J. 2021; 42(9):896.
2. Bhat T. Expert Rev Cardiovasc Ther. 2013; 11:55.
To cite this abstract in AMA style:
Merola J, Ogdie A, Kavanaugh A, Leibowitz E, Rampakakis E, Funk R, Nantel F, Lavie F, Rowland K, Soriano E. Longitudinal Evaluation of Neutrophil-to-Lymphocyte Ratio in Guselkumab-Treated Patients with Psoriatic Disease and Levels of Systemic Inflammation Associated with Elevated Cardiovascular Risk: Post-hoc Analysis of 4 Phase 3, Randomized, Controlled Studies [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/longitudinal-evaluation-of-neutrophil-to-lymphocyte-ratio-in-guselkumab-treated-patients-with-psoriatic-disease-and-levels-of-systemic-inflammation-associated-with-elevated-cardiovascular-risk-post-h/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/longitudinal-evaluation-of-neutrophil-to-lymphocyte-ratio-in-guselkumab-treated-patients-with-psoriatic-disease-and-levels-of-systemic-inflammation-associated-with-elevated-cardiovascular-risk-post-h/