Session Information
Date: Monday, October 22, 2018
Title: 4M094 ACR/ARHP Abstract: Health Services Research I: Focus on Big Data (1887–1892)
Session Type: ACR/ARHP Combined Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose:
Rheumatoid Arthritis (RA) is a complex systemic inflammatory disease with variable course that is difficult to precisely predict. Deep Learning (DL), a branch of artificial intelligence, has become state of the art in longitudinal predictions. It is unknown whether DL can be used to prognosticate RA outcomes. We aimed to use structured data from electronic health records (EHR) to build a deep learning model that would predict future RA disease activity.
Methods:
Data were derived from rheumatology clinics at two distinct health systems (an university health center and public safety net hospital) with different EHR platforms. We extracted structured data including demographics, diagnoses, medications, and prior disease activity measured by the Clinical Disease Activity Index (CDAI). We developed a multivariate longitudinal DL method to predict disease activity, grouped as controlled disease activity (low or remission) vs. uncontrolled (moderate or high), for RA patients at their next rheumatology clinic visit. We compared the predicted disease activity from our model to actual CDAI scores and calculated Area Under the Receiver Operating Characteristic curve (AUROC). Performance was compared to predictions based on the population-level likelihood of each outcome category (Baseline 1) as well as the probability of switching outcome from the prior visit (Baseline 2). We evaluated whether the model developed in one health system was generalizable to a second and assessed model interoperability strategies.
Results:
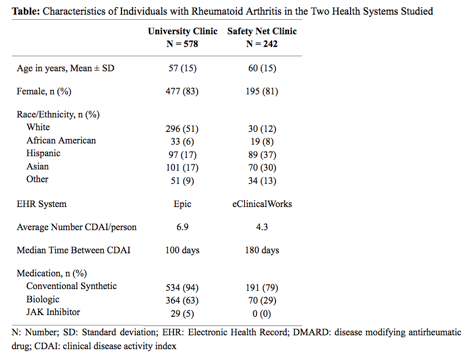
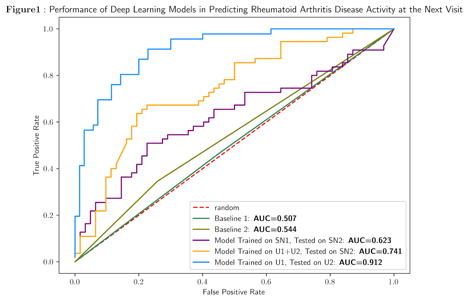
The university and safenet clinics were substantially different (Table). At the university hospital, the model was trained on a subset of patients (U1,n=462) and then tested on a separate group (U2,n=116) and reached an AUC of 0.91 (Figure). When trained on all patients at the university, the model generalized well (AUC 0.74) to a cohort of patients from the safety net hospital (SN2,n=117) and outperformed a model trained on a separate cohort of patients (SN1,n=125) from the safety net (AUC=0.63). In both settings the deep learning models outperformed baselines.
Conclusion:
We built accurate, generalizable longitudinal DL models to forecast patient outcomes on populations that only number in the hundreds. Our findings suggest that the RA model can be shared across hospitals with different EHR systems and diverse patient populations. Testing of artificial intelligence models in clinical practice is warranted to evaluate their usefulness in helping clinicians and patients prognosticate RA outcomes or simulate outcome trajectories under different treatment scenarios.
To cite this abstract in AMA style:
Norgeot B, Glicksberg B, Lituiev D, Trupin L, Gianfrancesco M, Butte A, Schmajuk G, Yazdany J. Longitudinal Deep Learning on Electronic Health Record Data to Predict Future Rheumatoid Arthritis Disease Activity [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/longitudinal-deep-learning-on-electronic-health-record-data-to-predict-future-rheumatoid-arthritis-disease-activity/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/longitudinal-deep-learning-on-electronic-health-record-data-to-predict-future-rheumatoid-arthritis-disease-activity/