Session Information
Date: Tuesday, November 14, 2023
Title: (2257–2325) SLE – Diagnosis, Manifestations, & Outcomes Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Antinuclear antibodies (ANAs) are a hallmark of systemic lupus erythematosus (SLE) and also a marker of subclinical autoimmunity. While a seminal study using the US Department of Defense Serum Repository suggested a progressive accumulation of autoantibodies before the onset of SLE, there have been no large-scale studies to assess changes in ANA titers within individuals over time. We performed this study to characterize longitudinal, intra-individual variation in ANAs in persons with SLE or other ANA-associated rheumatic diseases, as well as ANA-positive persons without rheumatic disease, in a large healthcare system in the Midwestern US.
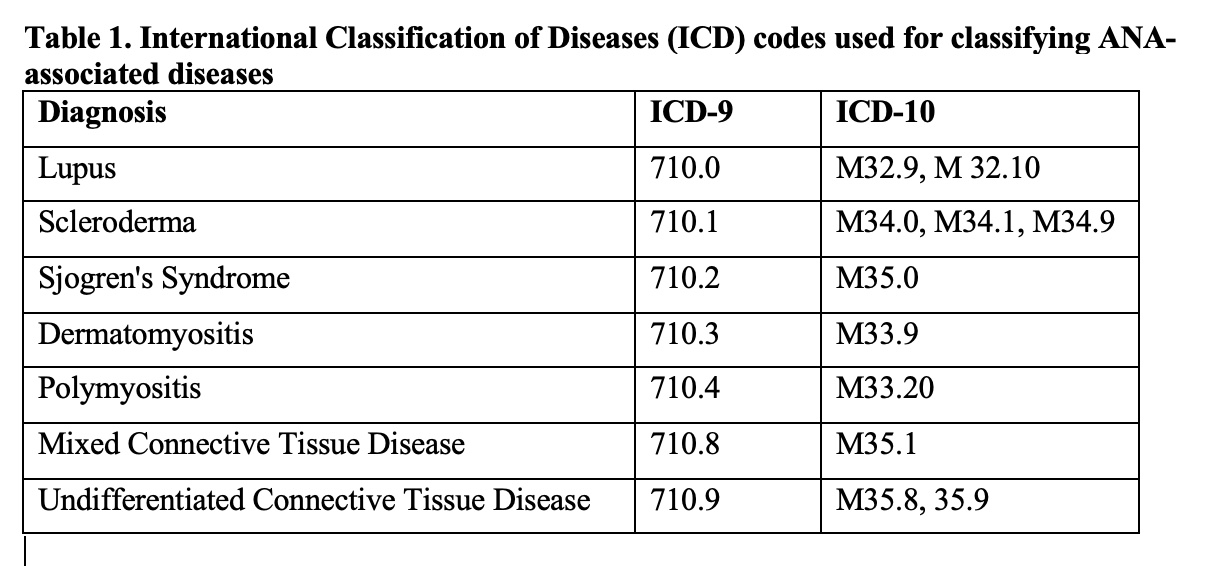
Methods: We performed an exploratory analysis of electronic health record data from an academic health system between 1999-2020. Among the patients with at least one positive ANA, we screened their electronic medical records for ANA-associated rheumatic disease diagnoses, based on International Classification of Diseases (ICD) coding. We categorized the study population into four groups:(1) SLE according to ICD coding; (2) A “validated” SLE subset of patients enrolled in our institutions IRB-approved Lupus Cohort; (3) “Other” ANA-associated rheumatic disease, and (4) ANA-positive controls without a history of ANA-associated rheumatic disease. The ICD codes utilized are listed in Table 1.
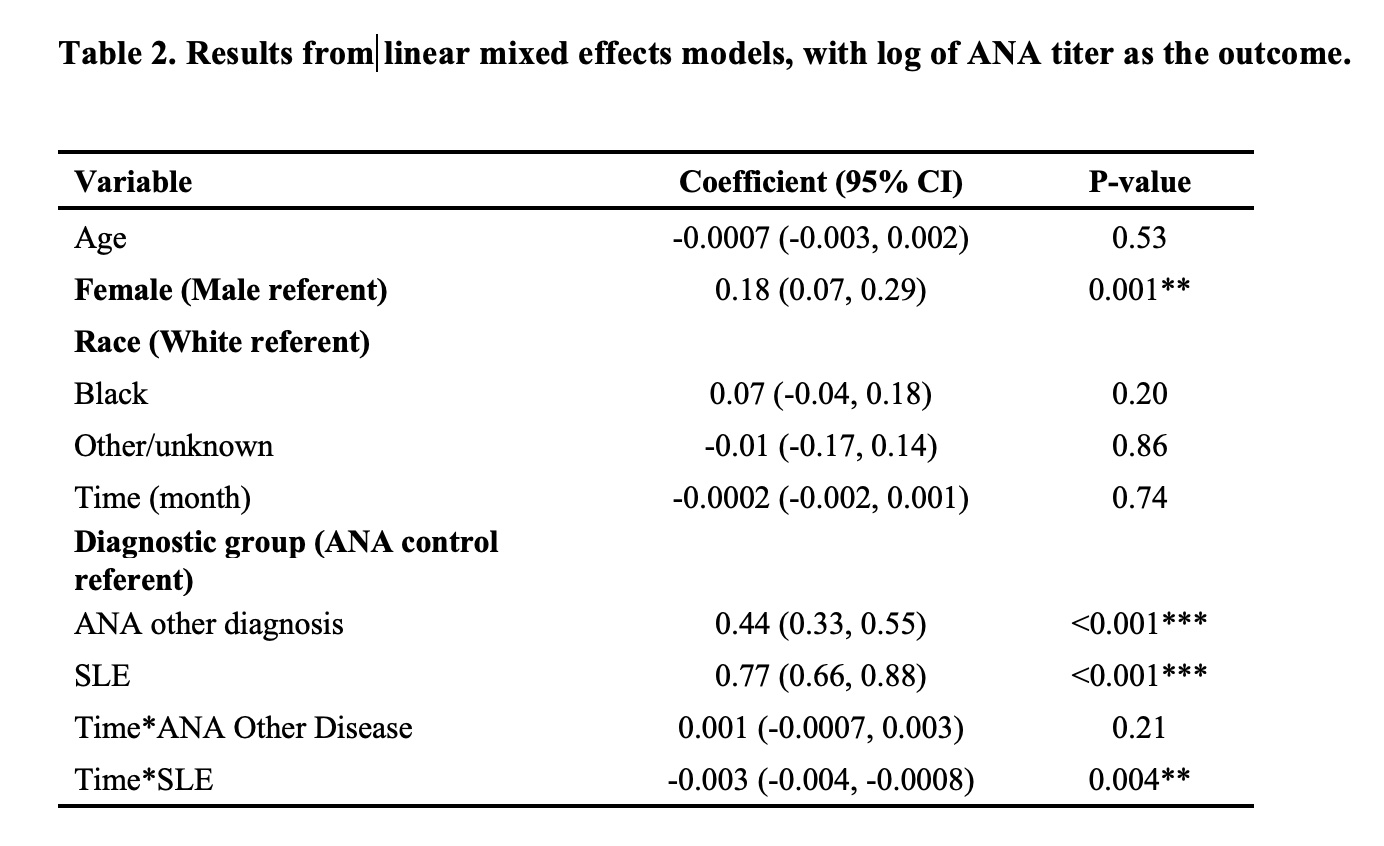
For group comparisons, ANOVA was utilized for continuous variables, and chi-square test for categorical variables. Multivariable generalized linear mixed effects models were utilized for analyses with ANA positivity (binary) as the outcome. For analyses with ANA titer strength (magnitude of positivity) as the outcome, linear mixed effects models were used. As laboratory measurement of ANA titers is based on the detection of”doubling” of levels (eg, 1:80, 1:160, 1:320, etc.), we took a log transformation on the ANA titer to normalize the skewness in the distribution of the variable.
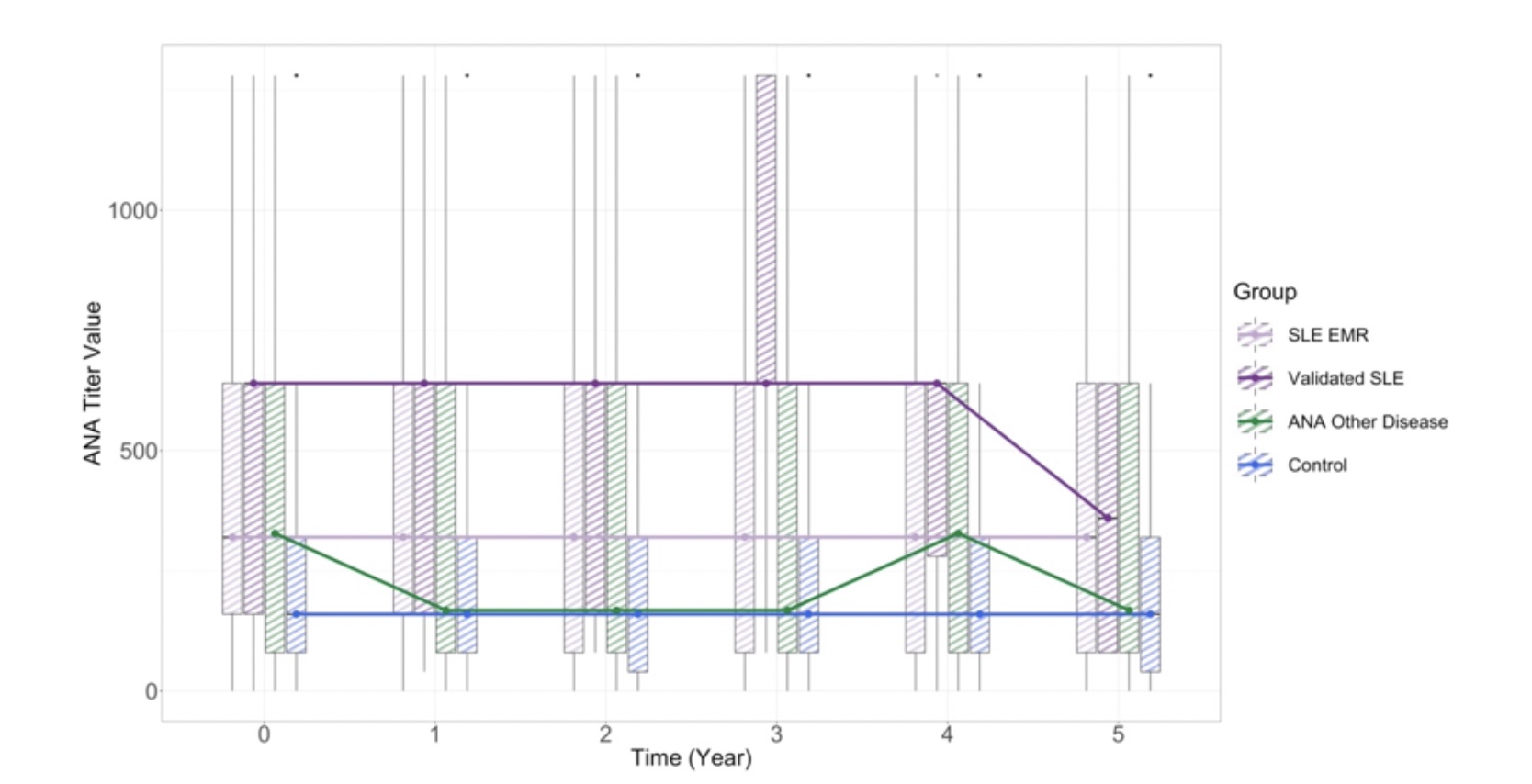
Results: SLE patients had a higher odds of a positive ANA titer compared to other ANA-associated rheumatic diseases [OR 2.05, 95% CI(1.77, 2.37)] (Figure 1). Compared to the ANA+ control group, the average ANA titer magnitude for the ANA-associated rheumatic diseases group was 0.44 units [95% CI (0.33, 0.55)] higher on the log scale controlling for age, sex and time, and 0.77 units [95% CI (0.66, 0.88)] higher for the SLE group, with both statistically significant (p< 0.001) (Table 2).
Longitudinally, in the validated SLE group, the likelihood of having a positive ANA decreased by 0.3% with each month [OR 0.997,95% CI (0.995, 0.998)]. ANA titer strength significantly decreased over time in the SLE group; after one year, the magnitude of ANA titer among patients in the SLE group was 0.033 units on the log scale lower than patients in the control group (95% CI 0.01, 0.04, p< 0.01).
Conclusion: Based on this large longitudinal dataset, ANA titers may be more dynamic than previously accepted in patients with SLE and ANA+ rheumatic diseases. Even in SLE, where titers were on average higher than for other ANA-associated diseases, declining ANA titers are seen over time, which might reflect aspects of disease states or flare risk that are beginning to be appreciated given accumulating evidence in other longitudinal studies.
To cite this abstract in AMA style:
Littlejohn E, Kong L, Wang L, Somers E. Longitudinal ANA Titers in SLE and ANA-associated Rheumatic Diseases [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/longitudinal-ana-titers-in-sle-and-ana-associated-rheumatic-diseases/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/longitudinal-ana-titers-in-sle-and-ana-associated-rheumatic-diseases/