Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Upadacitinib (UPA), an oral, JAK1-selective inhibitor showed efficacy over 12 weeks (wks) in patients (pts) with moderately to severely active rheumatoid arthritis (RA) and inadequate response to csDMARDs (SELECT-NEXT).1
We assessed safety and efficacy of UPA through Wk60 in an ongoing extension of the phase 3 SELECT-NEXT study.
Methods: Pts received once-daily (QD) UPA at 15 mg (UPA15), 30 mg (UPA30) or placebo (PBO) for 12 wks on stable background csDMARDs. From Wk12, the start of a long-term blinded extension, pts initially randomized to PBO at BL were switched to UPA15mg or 30mg per pre-specified assignment at BL. Pts randomized to UPA continued their assigned dose. No dose adjustments of UPA were allowed; however, starting at Wk24, adjustments to background RA medications were permitted. Sites/subjects remain blinded to UPA dose throughout the extension period. Efficacy data up to Wk60 are reported “As Observed”. Adverse events (AE) per 100 pt yrs (PY) are summarized based on a cut-off date of Mar 22 2018.
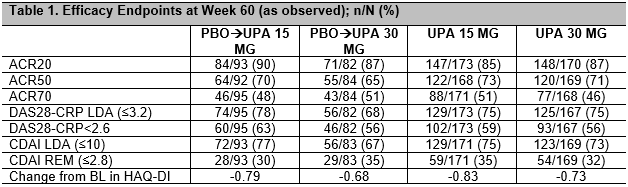
Results: 611/661 (92%) pts completed Wk12 and continued on to the extension. By the safety data cut-off date, 125/611 (20%) had discontinued study drug, 50 (8.2%) due to an AE, and 10 (1.6%) discontinued due to lack of efficacy. Cumulative exposure was 393.3 PYs and 372.4 PYs for UPA15 and UPA30 respectively. Based on As Observed analysis, for pts who continued on UPA15 (262/310 [85%]) and UPA30 (243/301 [81%]), clinical and functional outcomes continued to improve or were maintained through Wk60, with 59% and 56% of pts achieving DAS28-CRP < 2.6 and 35% and 32% achieving CDAI remission (≤2.8) with UPA 15 and 30 mg, respectively (Table 1). Pts who switched from PBO to UPA15 or UPA30 showed comparable efficacy to those initially randomized to UPA. The most frequently reported AEs were nasopharyngitis, urinary tract infection, upper respiratory tract infection, bronchitis, blood creatine phosphokinase increased, alanine aminotransferase increased, herpes zoster (HZ) and nausea. Most frequent AEs (≥0.8/100PYs) leading to premature study drug discontinuation were pneumonia, transaminase elevations, HZ and pyrexia. Event rates (E/100PYs) were numerically higher in UPA30 vs UPA15 for serious AE, AE leading to discontinuation, serious infections, HZ and malignancies, and were similar in UPA15 and UPA30 for adjudicated major adverse cardiovascular events and venous thromboembolic events (Table 2).
Conclusion: UPA15mg and 30mg on background csDMARD therapy demonstrated consistent efficacy and safety over 60 weeks in RA patients with inadequate response to csDMARDs. Both doses of UPA showed a similar efficacy profile at week 60, with numerically higher rates for certain safety events noted in the UPA30 group. An integrated safety analysis of upadacitinib across the phase 3 program is required to fully characterize the benefit:risk of UPA in RA.
To cite this abstract in AMA style:
Burmester G, Van den Bosch F, Bessette L, Kivitz A, Li Y, Friedman A, Pangan A, Camp H, Kremer J. Long-Term Safety and Efficacy of Upadacitinib in Patients with Rheumatoid Arthritis and an Inadequate Response to CsDMARDs: Results at 60 Weeks [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/long-term-safety-and-efficacy-of-upadacitinib-in-patients-with-rheumatoid-arthritis-and-an-inadequate-response-to-csdmards-results-at-60-weeks/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-safety-and-efficacy-of-upadacitinib-in-patients-with-rheumatoid-arthritis-and-an-inadequate-response-to-csdmards-results-at-60-weeks/