Session Information
Date: Monday, November 14, 2022
Title: Abstracts: Epidemiology and Public Health I: Risk Factors and Outcomes
Session Type: Abstract Session
Session Time: 9:00AM-10:30AM
Background/Purpose: Approximately 40% of adult patients with SLE develop LN, which can lead to end-stage kidney disease (ESKD).1 Renal response is used in clinical trials as a measure of treatment efficacy in patients with LN; however, there are various definitions of renal response and limited evidence to support whether it accurately predicts long-term renal outcomes. A study using data from the Hopkins Lupus Cohort showed that a real-world modified version of the primary efficacy renal response (mPERR; without requirement of tapering of oral steroid therapy) endpoint of the BLISS-LN study (NCT01639339) accurately predicted long-term renal outcomes.2
This study primarily assessed the association between mPERR status at 2 years (2Y) post-biopsy-proven LN and long-term renal survival (no ESKD or death) among patients with LN from the University of Toronto Lupus Cohort (TLC) database, a prospective, longitudinal cohort of patients with SLE.
Methods: This retrospective observational study (GSK Study 212866) used data from eligible adult patients with biopsy-proven class III, IV, V, III/V, or IV/V LN within the TLC. Patients were followed up from 2Y post-biopsy until censoring. Primary and secondary endpoints included the associations of mPERR status with long-term renal survival, and survival without moderate/severe chronic kidney disease (CKD; defined as absence of ≥2 new, consecutive eGFR < 60 ml/min/1.73 m2 occurrences recorded ≥3 months apart).
Patients were classified as mPERR or no mPERR at 2Y post-biopsy. mPERR was defined as proteinuria ≤0.7 g/24 h and an estimated glomerular filtration rate (eGFR) ≤20% below biopsy value or ≥60 ml/min/1.73 m2. Long-term renal survival was defined as the absence of ESKD (defined as eGFR < 30 ml/min/1.73 m2, dialysis, or transplant) or death, assessed during the follow-up period. Kaplan-Meier plots and log-rank tests were used to compare survival outcomes by mPERR status.
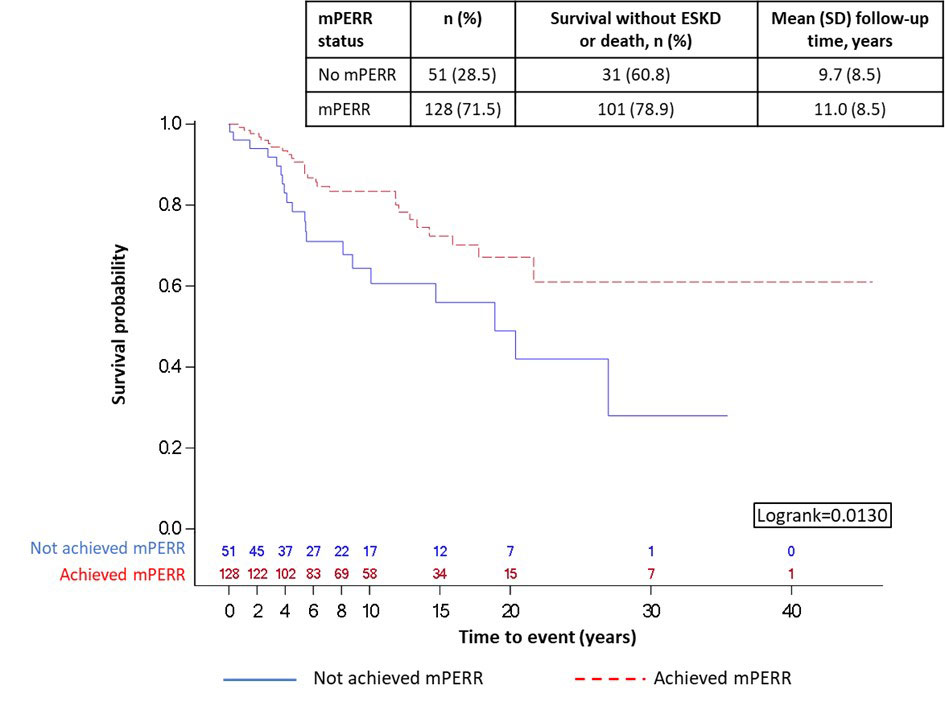
Results: In total, 179 patients were included in the ESKD analysis, with a mean (standard deviation) age of 34.2 (11.3) years at biopsy; class IV LN was the most frequent (n=66, 36.9%). At 2Y post-biopsy, 128 (71.5%) patients achieved mPERR, while 51 (28.5%) did not. Achieving mPERR was associated with an increased likelihood of long-term renal survival versus not achieving mPERR (p=0.0130; Figure 1).
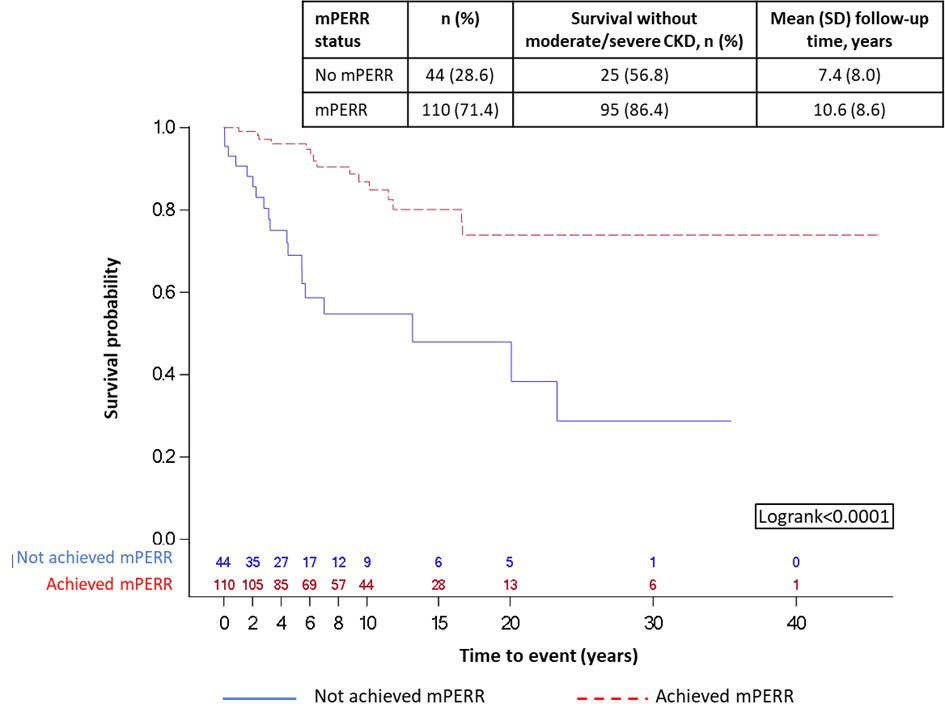
For long-term survival without moderate/severe CKD analysis, 154/179 patients were included; 25 had moderate/severe CKD prior to 2Y post-biopsy and were excluded. At 2Y post-biopsy, 110 (71.4%) patients achieved mPERR, while 44 (28.6%) did not. Achieving mPERR was associated with an increased likelihood of long-term survival without moderate/severe CKD versus not achieving mPERR (p< 0.0001; Figure 2).
Conclusion: Achieving mPERR at 2Y post-biopsy was associated with improved long-term renal survival and survival without moderate/severe CKD. This adds to the existing body of evidence that mPERR status is a suitable predictor of long-term renal outcomes within different patient populations and validates the need for sustained response to therapy.
Funding: GSK
References
1Mahajan A, et al. Lupus. 2020;29:1011–20
2Petri M, et al. Arthritis Rheumatol. 2020;72 (suppl 10)
Follow-up period is the time after mPERR assessment (year 0 in the figure is 2Y post-biopsy). Beyond year 15, patient sample sizes were small so should be interpreted with caution.
Follow-up period is the time after mPERR assessment (year 0 in the figure is 2Y post-biopsy). Beyond year 15, patient sample sizes were small so should be interpreted with caution.
To cite this abstract in AMA style:
Urowitz M, Georgiou M, Su J, MacKinnon A, Green Y, Gairy K, Levy R, Juliao P. Long-term Renal Survival of Patients with LN by Renal Response Status in the Toronto Lupus Cohort [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/long-term-renal-survival-of-patients-with-ln-by-renal-response-status-in-the-toronto-lupus-cohort/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-renal-survival-of-patients-with-ln-by-renal-response-status-in-the-toronto-lupus-cohort/