Session Information
Date: Tuesday, November 15, 2016
Title: Rheumatoid Arthritis – Clinical Aspects IV: Managing Patients in Remission
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: In a pragmatic, randomized, open label strategy study, non inferiority of a disease activity guided dose reduction (DR) strategy of adalimumab or etanercept compared to usual care (UC; non tapering tight control) was demonstrated after 18 months. Long term effects of this strategy are however unknown. We assessed whether the initial 18 months effects were sustained up to year 3 with regard to disease control, functioning, quality of life, radiographic outcome and cost (effectiveness).
Methods : In the intervention phase (months 0-18), patients were randomized to DR (stepwise TNFi interval increase until flare or discontinuation) or UC1. Consenting completers of this phase were included in the extension phase (months 18-36). Treatment in both groups converged to protocolized tight control and allowed dose optimization (Fig. 1). Intention-to-treat analyses were done on flare, medication use, disease activity (DAS28-CRP), functioning (HAQ-DI), quality of life (EQ5D-5L), adverse events (AE), radiographic progression (Sharp-Van der Heijde, SvdH), and costs.
Results : 172 (115 DR; 57 UC) were included in the extension phase (Fig. 2). Cumulative incidences of major flare were 10% and 12% (-2%, 95% CI -8 to 15) in the DR and UC group in the extension phase, and 17% and 14% (3%, 95% CI -9 to 13) from 0-36 month. In the DR group, 33/115 (29%, 95% CI 21 to 38%) still had successfully reduced their TNFi dose at 36 months, and 19/115 (17%, 95% CI 10 to 25%) discontinued TNFi at 36 months. In the UC group, 32/49 (65%, 95% CI 50 to 78%) attempted dose reduction in the extension phase of whom 19/49 (39%, 95%CI 25 to 54%) had successfully tapered and 7/49 (14%, 95% CI 1 to 27%) discontinued TNFi at 36 months. Mean DAS28-CRP, HAQ-DI, SvdH and EQ5D-5L remained stable over 36 months and did not differ significantly between groups (Fig. 3). Over 3 years, mean cost saving was −€13,451 (95% CI −€9,701 to−€17,198) per patient, which, with the small gain in QoL, resulted in a dominant cost-effectiveness ratio (€1.9 million savings per saved QALY).
Conclusion: Safety and efficacy of disease activity guided dose reduction of TNFi in RA patients are maintained up to three years. No difference in radiographic progression was found. Cost savings were considerable, no other benefits of tapering could be demonstrated. Reference Van Herwaarden N et al. BMJ 2015
Figure 1. Study conduct 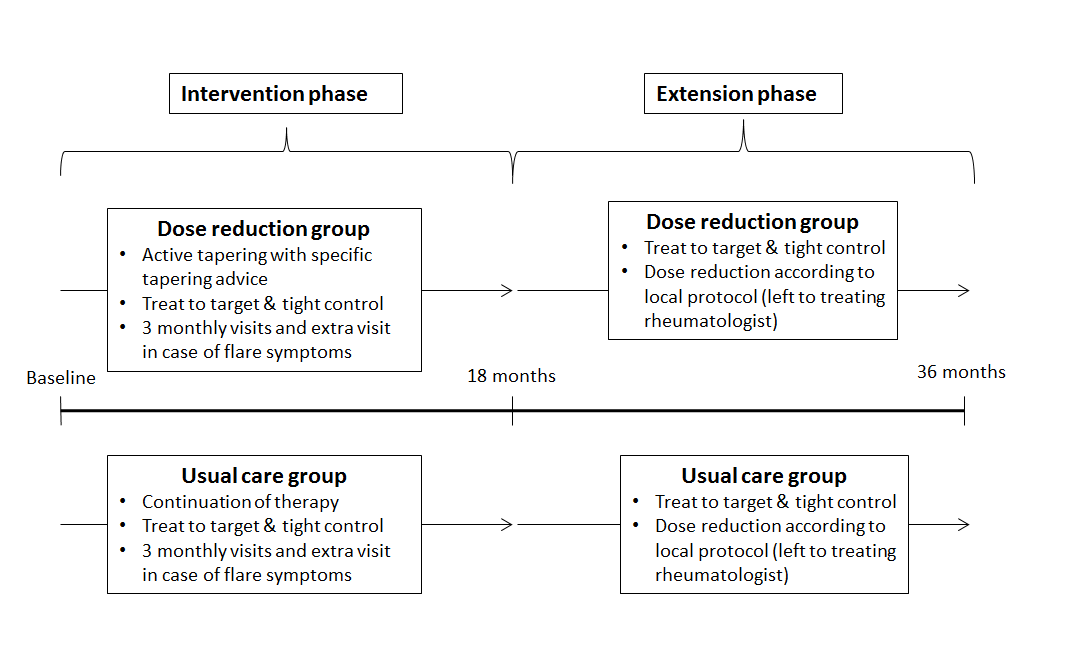 Figure 2. Flow chart
Figure 2. Flow chart 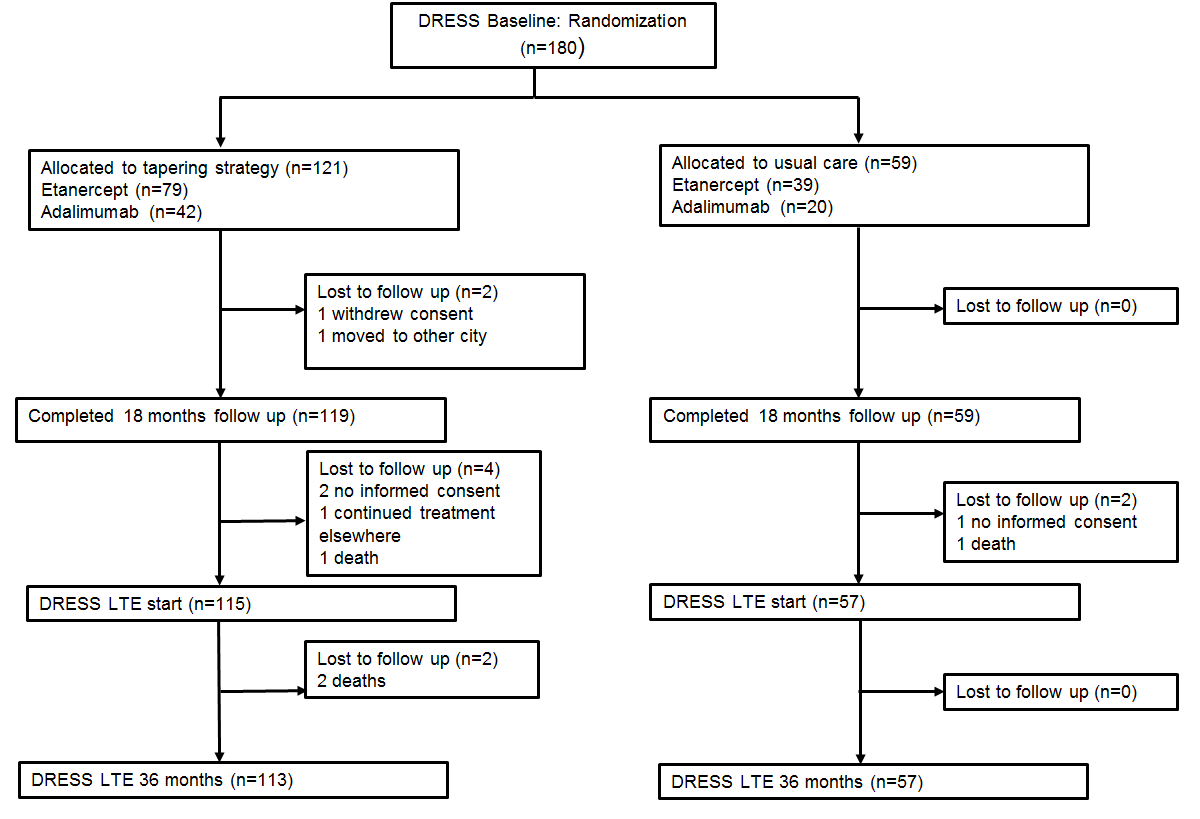 Figure 3. Mean A: Disease activity (measured with DAS28-CRP) B: Functioning (measured with HAQ-DI) C: Quality of life (measured with EQ5D-5L)
Figure 3. Mean A: Disease activity (measured with DAS28-CRP) B: Functioning (measured with HAQ-DI) C: Quality of life (measured with EQ5D-5L)
To cite this abstract in AMA style:
den Broeder AA, Bouman CAM, van den Hoogen FHJ, Fransen J, van Vollenhoven RF, Bijlsma JWJ, van der Maas A, van Herwaarden N. Long-Term Outcomes after Disease Activity Guided Tapering of Tumor Necrosis Factor Inhibition in Rheumatoid Arthritis: 3 Year Data of a Randomised Controlled Pragmatic Non Inferiority Strategy Study [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/long-term-outcomes-after-disease-activity-guided-tapering-of-tumor-necrosis-factor-inhibition-in-rheumatoid-arthritis-3-year-data-of-a-randomised-controlled-pragmatic-non-inferiority-strategy-study/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-outcomes-after-disease-activity-guided-tapering-of-tumor-necrosis-factor-inhibition-in-rheumatoid-arthritis-3-year-data-of-a-randomised-controlled-pragmatic-non-inferiority-strategy-study/

