Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Tofacitinib is a pan-JAK inhibitor that demonstrated safety and efficacy in a 12 week open label trial of 10 subjects with refractory dermatomyositis (NCT03002649). Long term disease activity and safety is unknown in subjects receiving tofacitinib.
Methods: To provide long-term safety and efficacy data in dermatomyositis subjects in the study, subjects who completed up to week 12 were eligible to receive tofacitinib XR 11mg daily in a long term study extension. Safety and efficacy using the validated ACR/EULAR Myositis Response Criteria, as measured by the Total Improvement Score (TIS) and Cutaneous Dermatomyositis Activity and Severity Index (CDASI) was assessed every 4 weeks until 20 weeks. Following this, subjects remained on drug and returned for a 42 week and 68 week visit.
Results: 7 of 10 (70%) of patients entered the long term extension of the study drug. There were no serious adverse events to date. 6 of 7 (86%) continued to meet the IMACS DOI at 68 weeks. The median Total Improvement Score (TIS) was 37.5 [30, 37.5] at 42 weeks, and 25 [22.5, 37.5] at 68 weeks. There continued to be a statistically significant change in mean CDASI when compared to baseline at all time points during the extension period. The mean baseline CDASI was 25.4 + 15 which dropped to 3.85 + 2.41 (p=0.008) by week 42, and 5.43 + 2.51 by week 68 (p=0.01). Two subjects required additional immunosuppression with low dose methotrexate during the long term extension period. No patients required any high dose steroid therapy or admission to the hospital for a flare.
Conclusion: Tofacitinib continues to be safe and well tolerated in the long term open label extension of the study. There were no serious adverse events or study discontinuation related to tofacitinib. These data support further investigation of JAK inhibitors in dermatomyositis in a randomized placebo controlled study.
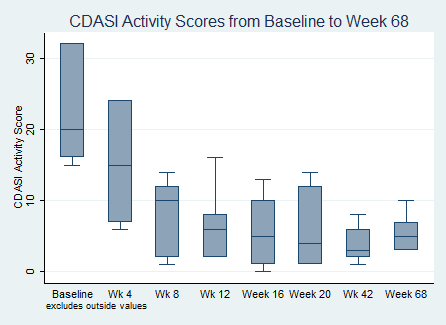 CDASI Activity Scores from Baseline to Week 68
CDASI Activity Scores from Baseline to Week 68
To cite this abstract in AMA style:
Paik J, Albayda J, Tiniakou E, Purwin G, Koenig A, Christopher-Stine L. Long Term Open Label Extension of Study of Tofacitinib in Refractory Dermatomyositis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/long-term-open-label-extension-of-study-of-tofacitinib-in-refractory-dermatomyositis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-open-label-extension-of-study-of-tofacitinib-in-refractory-dermatomyositis/
