Session Information
Session Type: Late-Breaking Abstracts
Background/Purpose: Cohort studies demonstrate that 11-83% of female patients receiving cyclophosphamide (CYC) for treatment of severe manifestations of SLE develop premature ovarian failure (POF), dependent largely on the cumulative dose and age. The co-administration of gonadotropin-releasing hormone agonists (GnRHa), such as leuprolide acetate (LA), appears to mitigate the risk of CYC-induced POF however each of these agents predisposes patients to decreased bone mineral density (BMD) through a resultant hypoestrogenemia. Uncertainty remains regarding the extent to which CYC- and GnRHa-associated BMD loss is restored, particularly in the setting of SLE.
Methods: Seventeen women with diffuse proliferative lupus nephritis (DPLN) enrolled in an ongoing pilot study of LA suppression during intermittent CYC therapy to preserve ovarian function received baseline and follow-up evaluation of BMD at using a Hologic 1000W (Waltham, MA). Patients were counseled to supplement with 600 mg calcium carbonate and 200 IU vitamin D twice daily. Additional risk factors for osteoporosis (OP) included: pulse methylprednisolone and oral prednisone therapy (n=17), race (5 Caucasian, 2 Asian, 1 Hispanic), nulliparity (n=6), cigarette smoking (n=3), and alcohol intake (n=3). Average age was 29.2 (range 15 to 37).
Results:
Table 1: Patients grouped by BMD status at baseline with measurements shown for 6 month, 1 year, 2-5 year, 5-10 year, 10-15 year, and 15-20 year follow-up intervals. One patient, OP at baseline, developed an OP-related fracture 11 years after initiation of LA/CYC therapy.
|
BMD at Baseline |
|
6 months |
1 year |
1-5 years |
5-10 years |
10-15 years |
15-20 years |
|
Normal |
Normal |
5 |
4 |
4 |
1 |
2 |
1 |
|
(n=9) |
Osteopenia |
2 |
1 |
0 |
1 |
0 |
0 |
|
|
Osteoporosis |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
Total |
7 |
5 |
4 |
2 |
2 |
1 |
|
Osteopenia |
Normal |
1 |
0 |
1 |
0 |
0 |
0 |
|
(n=6) |
Osteopenia |
4 |
4 |
4 |
2 |
1 |
0 |
|
|
Osteoporosis |
0 |
0 |
0 |
0 |
1 |
0 |
|
|
Total |
5 |
4 |
5 |
2 |
2 |
0 |
|
Osteoporosis |
Normal |
0 |
0 |
1 |
0 |
0 |
0 |
|
(n=2) |
Osteopenia |
2 |
0 |
0 |
1 |
0 |
0 |
|
|
Osteoporosis |
0 |
0 |
1 |
0 |
0 |
0 |
|
|
Total |
2 |
0 |
2 |
1 |
0 |
0 |
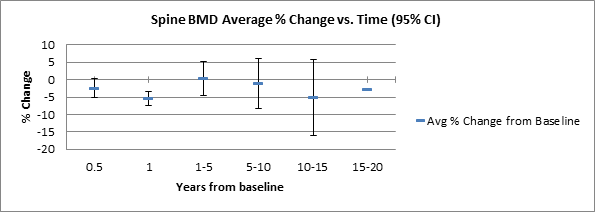
Fig. 2: Average percent change in BMD measured in L1-L4 vertebrae vs. time with 95% confidence intervals.
Conclusion: On average, BMD was initially restored following a nadir one year into treatment. Greatest early losses in BMD were seen in patients with normal BMD at entry. Though decreased bone mineralization has been shown in women with SLE even in the absence of exposure to CYC and LA, this therapy was not uniformly associated with long-term adverse effects on bone health in this population.
Disclosure:
N. Meier,
None;
M. A. Dooley,
None.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-evaluation-of-bone-mineral-density-change-in-women-receiving-depot-leuprolide-acetate-during-cyclophosphamide-therapy-for-severe-lupus-nephritis/