Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Radiographic joint damage progresses in 20-30% of rheumatoid arthritis (RA) patients despite fulfilling clinical remission criteria (1). Osteitis assessed on MRI is a well known predictor of subsequent radiographic bone damage progression (2). Therefore, targeting absence of osteitis combined with clinical remission may improve long-term radiographic outcomes. The purpose of the study was therefore: To investigate whether a 2-year MRI treat-to-target (MRI T2T) strategy targeting absence of osteitis combined with clinical remission, compared to a conventional T2T strategy targeting clinical remission only, could reduce radiographic joint damage progression over 5 years in RA patients.
Methods: IMAGINE-more was designed as a 3-year observational extension study of the 2-year IMAGINE-RA randomized clinical trial (3). IMAGINE-RA included 200 RA patients in clinical remission (DAS28-CRP< 3.2 and no swollen joints), with erosive disease (bone erosion on conventional radiography),and treated with conventional synthetic DMARDs (csDMARDs). The objective was to investigate whether a 2-year MRI T2T strategy targeting absence of osteitis combined with clinical remission (DAS28-CRP≤3.2 and no swollen joints) as compared to a conventional T2T strategy, targeting clinical remission only, could improve remission rates and prevent radiographic joint damage progression. If treatment target was not met, treatment was intensified stepwise starting with increment in csDMARDs and subsequently adding biologics. Participants in the IMAGINE-more extension study were managed in routine clinical practice in outpatient clinics. Clinical examinations and radiographs of hands and feet (also obtained at baseline, year 1 and 2 in IMAGINE-RA) were done year 3, 4 and 5. The primary endpoint was the proportion of patients with no radiographic progression (increase in total van der Heijde-modified Sharp score (vdHSS) ≤0) from baseline to year 5. Secondary endpoints were changes from baseline to 5 years in total vdHSS, vdHSS erosion and joint space narrowing (JSN) scores. Dichotomous endpoints were estimated by logistic regression, while median differences were calculated for the continuous outcome measures.
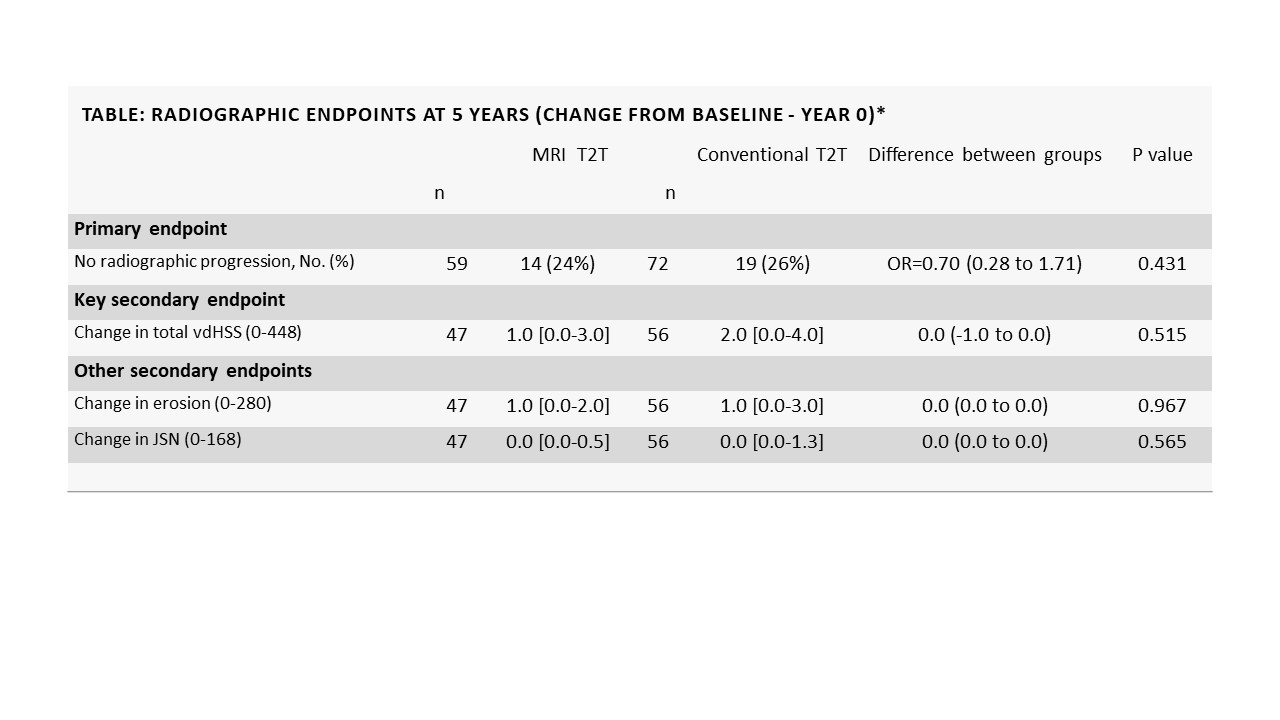
Results: Informed consent to participation in IMAGINE-more was obtained from 131 patients (59 from the originalMRI T2T group). Of these, 14 patients (24%) in the MRI T2T group and 19 patients (26%) in the conventional T2T group had no radiographic progression from baseline to year 5 (OR 0.70 [0.28 to 1.71]). As illustrated in the Table and Figure, the median progression in total vdHSS from baseline to 5 years was low, with no differences between treatment groups.
Conclusion: A 2-year combined MRI T2T and clinical T2T strategy, compared with a conventional clinical T2T strategy alone, did not result in reduced radiographic progression in the long term over 5 years in RA patients with erosive disease in clinical remission. In accordance with the primary results from the IMAGINE-RA trial, these findings do not support systematic use of MRI to guide treatment in RA patients in remission.
References: 1. Lillegraven et al. Ann Rheum Dis 2012 2. Brown et al. Arthritis Rheum 2008 3. Møller-Bisgaard et al. JAMA 2019
*In addition to the radiographic endpoints, the full IMAGINE-more study includes a clinical co-primary endpoint, and several secondary endpoints.
To cite this abstract in AMA style:
Møller-Bisgaard S, Hørslev-Petersen K, Ørnbjerg L, Ejbjerg B, Hetland M, Moeller J, Christensen R, Nielsen S, Glinatsi D, Boesen M, Stengaard-Pedersen K, Madsen O, Jensen B, Villadsen J, Hauge E, Hendricks O, Lindegaard H, Krogh N, Jurik A, Thomsen H, Østergaard M. Long-term Efficacy of a 2-year MRI Treat-to-target Strategy on Radiographic Joint Damage Progression in Rheumatoid Arthritis Patients in Clinical Remission – Five Year Follow-up of the IMAGINE-RA Randomized Clinical Trial [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/long-term-efficacy-of-a-2-year-mri-treat-to-target-strategy-on-radiographic-joint-damage-progression-in-rheumatoid-arthritis-patients-in-clinical-remission-five-year-follow-up-of-the-imagine/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-efficacy-of-a-2-year-mri-treat-to-target-strategy-on-radiographic-joint-damage-progression-in-rheumatoid-arthritis-patients-in-clinical-remission-five-year-follow-up-of-the-imagine/