Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Filgotinib (FIL) is an oral Janus kinase 1 preferential inhibitor for the treatment of moderate to severe active RA. A previous pooled analysis reported long-term safety and efficacy for FIL 200 mg (FIL200) vs FIL 100 mg (FIL100) in patients (pts) aged ≥ 65 and < 65 y.1 The objective of this analysis was to report updated long-term safety and efficacy in 4 subgroups of pts with RA (aged < 65 y, ≥ 65 y, < 65 y without cardiovascular [CV] risk, and ≥ 65 y or with CV risk), treated with FIL200 vs FIL100.
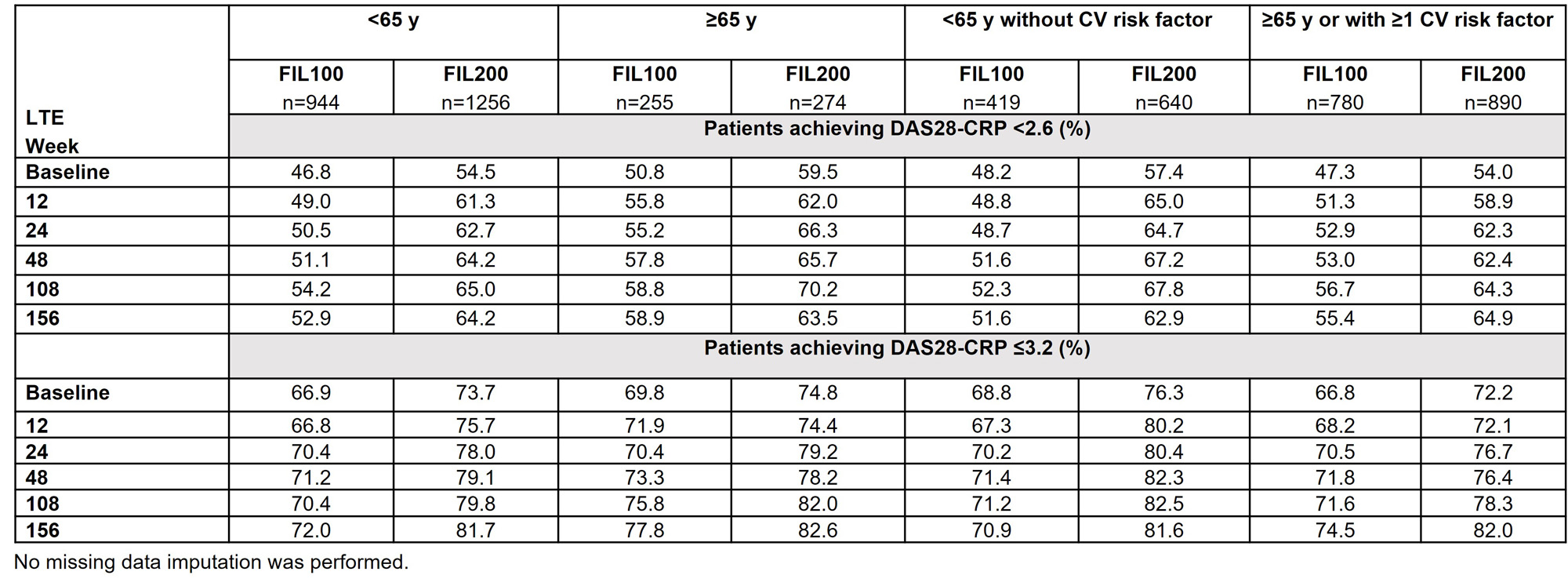
Methods: This post hoc analysis pooled data from Phase 2 (DARWIN 1–3; NCT01888874, NCT01894516, NCT02065700) and Phase 3 (FINCH 1–4; NCT02889796, NCT02873936, NCT02886728, NCT03025308) trials. Data for long-term extensions (LTEs) were as of May 2, 2022 (DARWIN 3), and May 6, 2022 (FINCH 4). Analyses were by age (< 65 vs ≥ 65 y) and subgroup (< 65 y without CV risk factor vs ≥ 65 y or ≥1 CV risk factor). CV risk factors were: family history of CV disease; history of dyslipidemia, diabetes mellitus or CV disease; hypertension, ischemic vascular conditions, peripheral vascular disease, extra-articular manifestations of RA; or ever smoked. The as-treated analysis set included data for pts receiving ≥1 FIL dose. Exposure-adjusted incidence rates (EAIRs)/100 patient-years of exposure (PYE) of selected adverse events (AEs), and % of pts achieving Disease Activity Score in 28 joints using C-reactive protein (DAS28-CRP) of < 2.6 or ≤ 3.2 through Week 156 in FINCH 4, are reported.
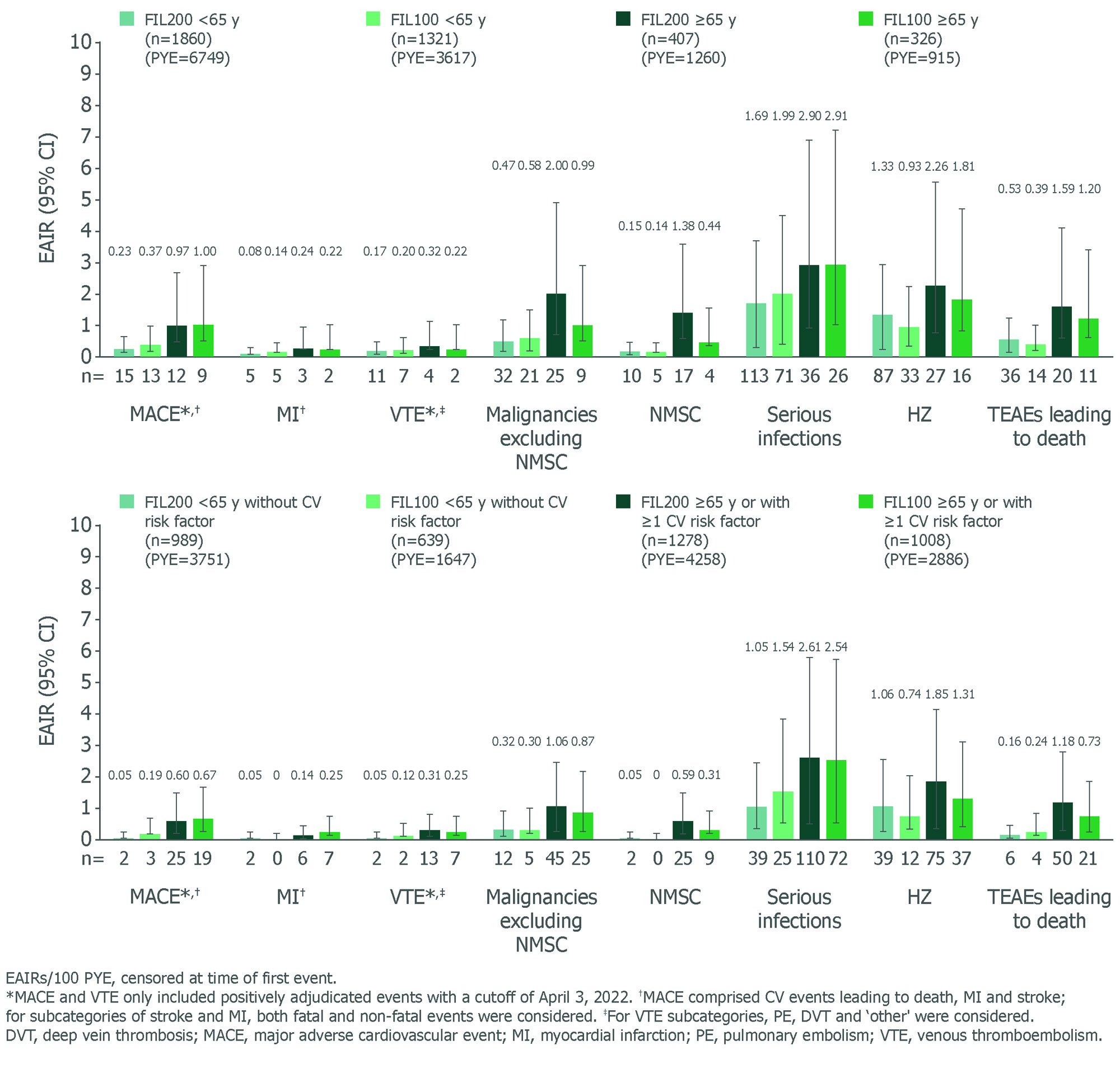
Results: Baseline characteristics by age have been reported.1 Baseline disease characteristics by age or CV risk factor were similar for disease severity and concurrent treatment. EAIRs for any treatment-emergent AEs (TEAEs) were generally higher in pts aged ≥ 65 (120.40) vs < 65 y (97.86), and higher in pts aged ≥ 65 y or with CV risk (120.45) vs pts aged < 65 y without CV risk (81.83). Pts aged ≥ 65 y, followed by the broader subgroup with CV risk factors (i.e. ≥ 65 y or ≥ 1 CV risk) had higher EAIRs of serious TEAEs and TEAEs leading to discontinuation vs other subgroups, indicating age is a key risk factor alongside other CV risk factors for developing AEs (data not shown). In pts aged ≥ 65 y, lower incidences of malignancies (excluding nonmelanoma skin cancer [NMSC]), NMSC, herpes zoster (HZ) and TEAEs leading to death were observed with FIL100 than FIL200 (Figure). In the broader subgroup with risk factors (i.e. aged ≥ 65 y or ≥ 1 CV risk factor), EAIRs of AEs were generally lower vs pts aged ≥ 65 y, indicating greater influence of age. EAIRs of AEs for subgroups (aged < 65 y without CV risk; ≥ 65 y or ≥ 1 CV risk) for FIL200 and FIL100 are shown (Figure). Rates of DAS28-CRP < 2.6 or ≤ 3.2 in all subgroups (FINCH 4) were maintained with FIL100 and FIL200 to Week 156 (Table).
Conclusion: This post hoc analysis (in pts ≥ 65 y or with ≥ 1 CV risk factor) suggests that age is an important risk factor in the evaluation of the FIL200 safety profile. Future studies are needed to address the relative contribution of age to traditional CV risk factors. In FINCH 4, efficacy was maintained in all subgroups.
Reference:
1. Buch M, et al. Arthritis Rheumatol 2022;74(Suppl 9):0281
To cite this abstract in AMA style:
Buch M, Gomez-Puerta J, Burmester G, Combe B, Rajendran V, Stiers P, Van Hoek P, Van Beneden K, Gottenberg J, Tanaka Y, Aletaha D, Westhovens R, Caporali R. Long-term Clinical Profile of Filgotinib in Patients with Rheumatoid Arthritis by Cardiovascular Risk Factors: A Post Hoc Subgroup Analysis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/long-term-clinical-profile-of-filgotinib-in-patients-with-rheumatoid-arthritis-by-cardiovascular-risk-factors-a-post-hoc-subgroup-analysis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-clinical-profile-of-filgotinib-in-patients-with-rheumatoid-arthritis-by-cardiovascular-risk-factors-a-post-hoc-subgroup-analysis/