Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Long-term data on anti-TNF treatment in patients with early axial spondyloarthritis (SpA) is scarce. The objective of this analysis was to assess the long-term clinical efficacy (up to 10 years of treatment) of a tumor necrosis factor (TNF) inhibitor etanercept (ETN) in patients with early axial spondyloarthritis, who participated in the long-term (until year 10) extension of the ESTHER (Etanercept vs. Sulfasalazine in Early Axial Spondyloarthritis Trial) trial (1).
Methods: In the previously reported ESTHER trial, patients with early active axial SpA [including both non-radiographic axial SpA (nr-axSpA) and radiographic axial SpA (r-axSpA)/ankylosing spondylitis (AS)] with a symptom duration of < 5 years and a positive MRI of the sacroiliac joints (SIJs) and/or the spine at baseline) were treated with ETN (n= 40) or sulfasalazine (SSZ) (n= 36) during the first year (1). At year 1, all patients who were not in remission continued with – or switched (in case of SSZ therapy) to – ETN for up to 10 years in total (1). Patients in remission discontinued their therapy and were followed-up until end of year 2; in case of remission loss, ETN was (re)-introduced and continued till the end of year 10.
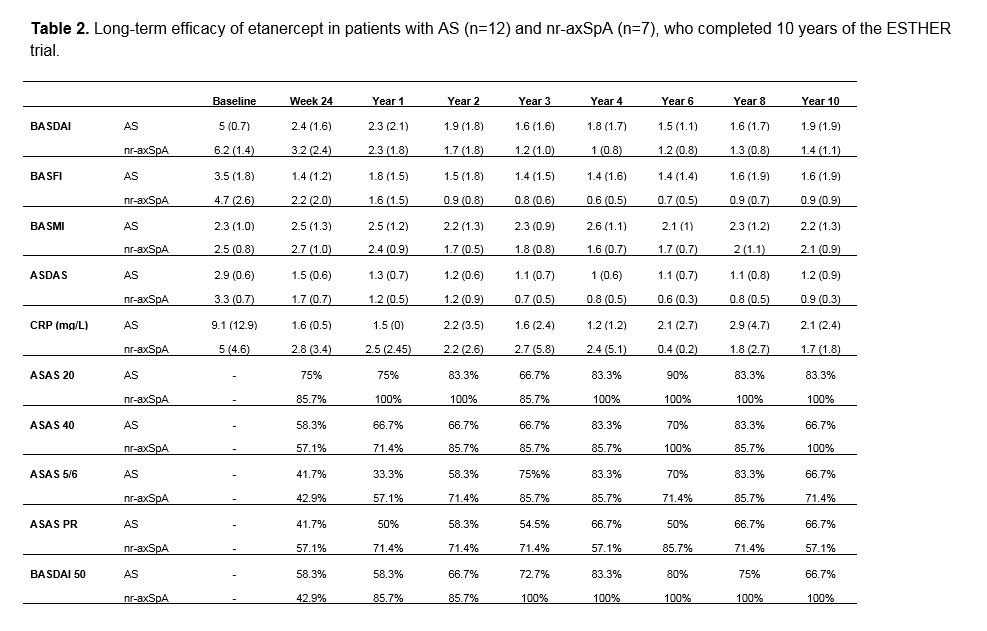
Results: Out of 76 initial patients, 25% (n=19, 12 with r-axSpA and 7 with nr-axSpA) completed year 10 of the study. At baseline, completers were significantly more often male and showed lower values of patient (PGA) and physician global assessments of disease activity (PhGA), ASDAS (Ankylosing Spondylitis Disease Activity Score), BASMI (Bath Ankylosing Spondylitis Metrology Index), and AS-QoL (Ankylosing Spondylitis Quality of Life Questionnaire) as compared to non-completers (Table 1). When analyzing clinical data of the completers, mean BASDAI, BASFI and ASDAS values were constantly < 2 during the follow up with no statistically significant differences between the r-axSpA and nr-axSpA sub-groups (Table 2, Figure 1B). In the entire group, a sustained clinical response was observed over 10 years of follow up (Figure 1A). A total of 39 serious adverse events were documented over the 10 years of the study, while six of them were seen as possibly associated with ETN treatment, which lead in five patients (one lymphoma, one sarcoidosis, one demyelinating neurological disease, one elevated liver enzymes and one recurrent minor infections) to an ETN discontinuation.
Conclusion: A sustained clinical response was shown over the 10 years of the study for the completers with comparable rates between r-axSpA and nr-axSpA. ETN was well tolerated across the entire treatment period and showed a good safety profile with no new safety signals.
Acknowledgements:
The ESTHER study was supported by an unrestricted research grant from Pfizer. Murat Torgutalp’s (MT) work at Charité – Universitätsmedizin was supported by an award from the Scientific and Technological Research Council of Turkey (TUBITAK).
References:
- Song IH, Hermann K, Haibel H, Althoff CE, Listing J, Burmester G, et al. Effects of etanercept versus sulfasalazine in early axial spondyloarthritis on active inflammatory lesions as detected by whole-body MRI (ESTHER): a 48-week randomised controlled trial. Ann Rheum Dis. 2011;70(4):590-6.
related parameters in study completers -n=19- -B-.
To cite this abstract in AMA style:
Proft F, Torgutalp M, Weiß A, Protopopov M, Rios Rodriguez V, Haibel H, Behmer O, Sieper J, Poddubnyy D. Long-term Clinical Outcome of Anti-TNF Treatment in Patients with Early Axial Spondyloarthritis: 10-year Data of the Etanercept vs. Sulfasalazin in Early Axial Spondyloarthritis Trial [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/long-term-clinical-outcome-of-anti-tnf-treatment-in-patients-with-early-axial-spondyloarthritis-10-year-data-of-the-etanercept-vs-sulfasalazin-in-early-axial-spondyloarthritis-trial/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-clinical-outcome-of-anti-tnf-treatment-in-patients-with-early-axial-spondyloarthritis-10-year-data-of-the-etanercept-vs-sulfasalazin-in-early-axial-spondyloarthritis-trial/