Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: One central mechanism believed to contribute to the pathogenesis of neuropsychiatric systemic lupus erythematosus (NPSLE) is temporary disruption of the blood brain barrier (BBB). Astrocytes are abundant glial cells in the brain that work with endothelial cells to regulate tight junctions, which maintain BBB integrity. Astrocyte end-feet localized along the BBB can sense and detect inflammatory cytokines and cells in the periphery; thus, the systemic inflammatory milieu present in SLE can lead to the activation of astrocytes, temporarily breaching the BBB and compromising the brain’s immune privilege. BBB dysfunction can also lead to the migration of immune cells into the brain and further activation of astrocytes and microglia, resulting in neuronal damage and subsequent cognitive defects. The MRL/lpr mouse is the most widely used spontaneous murine model of NPSLE. MRL/lpr mice show behavioral abnormalities such as cognitive deficits in the form of impaired memory (spatial recognition) as well as depressive-like behavior. Lipocalin-2 (LCN2) is an acute phase protein implicated in immune-mediated and neuroinflammatory diseases, including multiple sclerosis and traumatic brain injury. To examine a possible role of LCN2 in the pathogenesis of SLE we generated aMRL/lprLCN2 knockout (KO) mouse strain, and evaluated end organ disease, including neuropsychiatric, skin, and kidney disease. Furthermore, we focused on uncovering specific mechanisms by which LCN2 improves disease in lupus target organs.
Methods: Seventeen week old MRL/lpr-LCN2KO (n=15)and MRL/lprLCN2 wild type (n=16) mice underwent standardized behavioral testing, and were subsequently bled and sacrificed. Skin lesions were scored using a validated scoring protocol. Serum was tested for anti-dsDNA antibodies and IgG levels via ELISA. Skin and brain tissues were processed for immunofluorescent staining for T cells, B cells, macrophages, and neutrophils. Brain tissue was stained for NeuN (a neuronal marker) and glial fibrillary acidic protein (GFAP, an astrocyte activation marker).
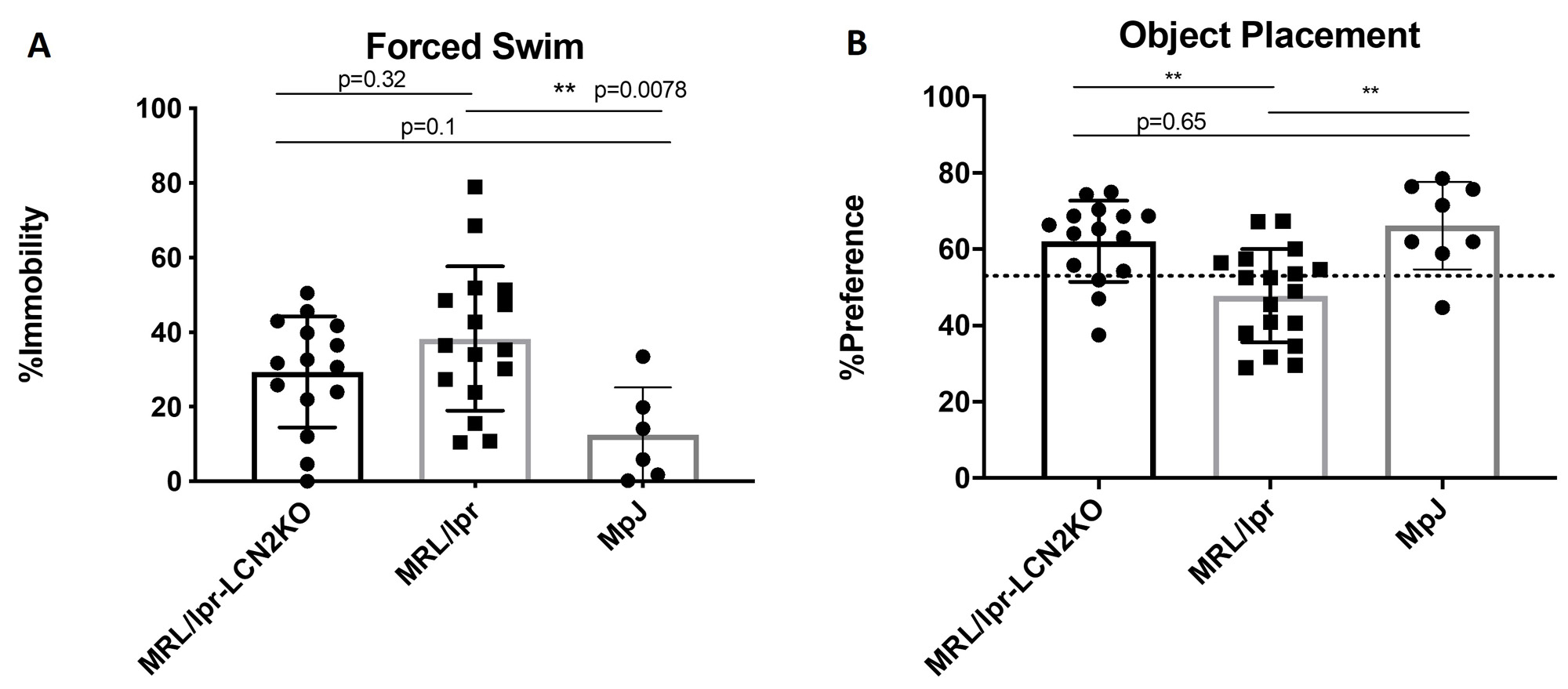
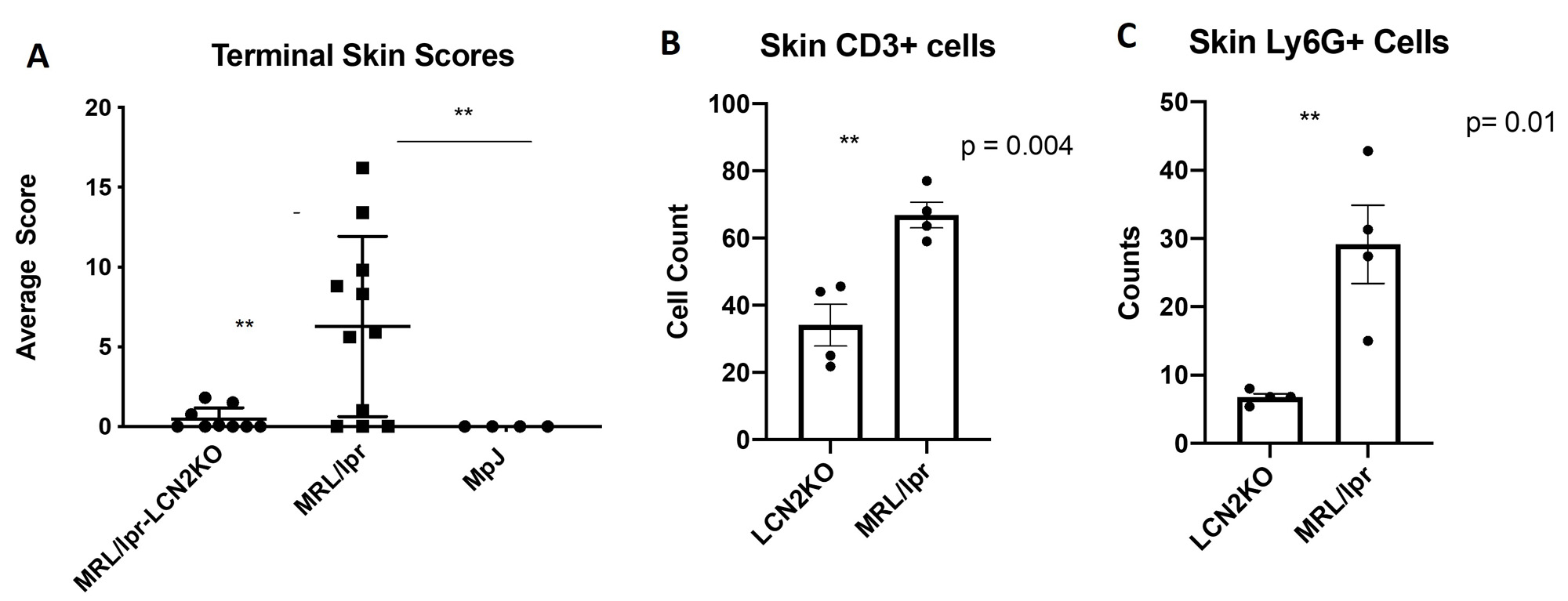
Results: We found that MRL/lpr-LCN2KO mice had significantly improved cognitive performance on spatial recognition memory and tests of behavioral despair, but unchanged levels of serum IgG and anti-dsDNA antibodies, compared to MRL/lpr mice. Additionally, we found that skin scores (macroscopic) in LCN2KO mice were significantly improved, together with a reduction in infiltrating T-cell and neutrophil counts. In the brain, we found diminished GFAP expression in MRL/lpr-LCN2KO mice, indicating reduced astrocyte activation. Moreover, NeuN expression was decreased inMRL/lprLCN2KO-derived hippocampal neurons, consistent with improved hippocampal neuron viability in LCN2 deficient mice.
Conclusion: LCN2 deficiency improves skin and neuropsychiatric disease in MRL/lpr lupus prone mice. While investigation of additional mechanisms is in progress, we found that skin disease is improved through reduction of infiltrating immune cells known to contribute to the severity of cutaneous lesions, while in the brain LCN2 deficiency reduces astrocyte activation and improves hippocampal neuron damage.
To cite this abstract in AMA style:
Putterman C, Mike E, Garcia S. Lipocalin-2 Promotes Cutaneous and Neuropsychiatric Disease in Murine Lupus [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/lipocalin-2-promotes-cutaneous-and-neuropsychiatric-disease-in-murine-lupus/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/lipocalin-2-promotes-cutaneous-and-neuropsychiatric-disease-in-murine-lupus/