Session Information
Date: Sunday, November 17, 2024
Title: Systemic Sclerosis & Related Disorders – Basic Science Poster I
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Pulmonary hypertension (PH) is a severe, progressive disorder characterized by elevated pulmonary artery pressures, right ventricular hypertrophy, and increased mortality which is a severe comorbidity of systemic sclerosis and other connective tissue diseases. We previously demonstrated that TNF-transgenic (TNF-Tg) mice develop a severe PH phenotype and that TNFR1 deficiency reverses this pathology. To explore the roles of cellular interactions contributing to PH in this model, we assessed single-cell RNA expression in TNF-Tg mice crossed with mice lacking TNFR1 or TNFR2. We found dramatic changes in cell populations between groups, and sought to investigate the cellular communication among immune, endothelial, and stromal populations in the lung.
Methods: TNF-Tg mice were crossed with mice deficient in either TNFR1(TNFR1KO) or TNFR2 (TNFR2KO). Lungs from wild type, TNF-Tg, TNF-Tg /TNFR1KO and TNF-Tg /TNFR2KO mice (n = 5–6) were harvested at 4.5 months of age for histological analysis and single cell RNA sequencing (scRNAseq). The scRNAseq data was analyzed in Seurat v.4.9. Differential expression and pathway analysis were performed and visualized using Seurat and enrichR. Intercellular communication was investigated using NicheNet.
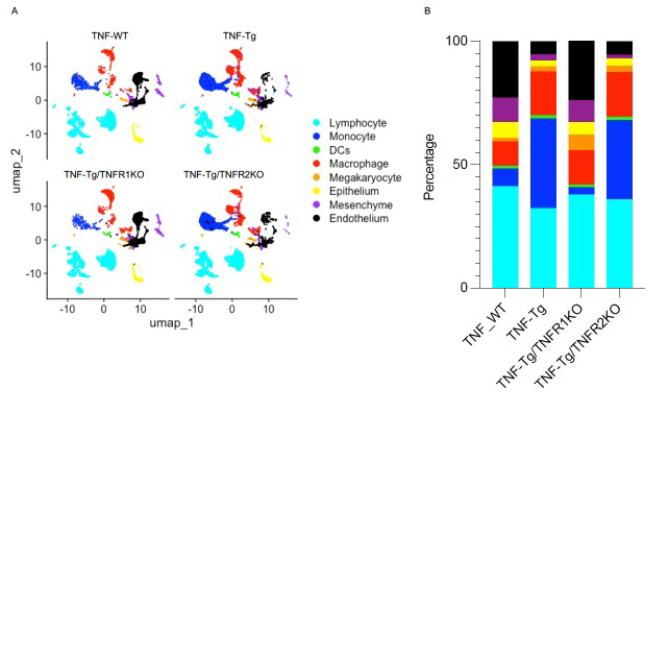
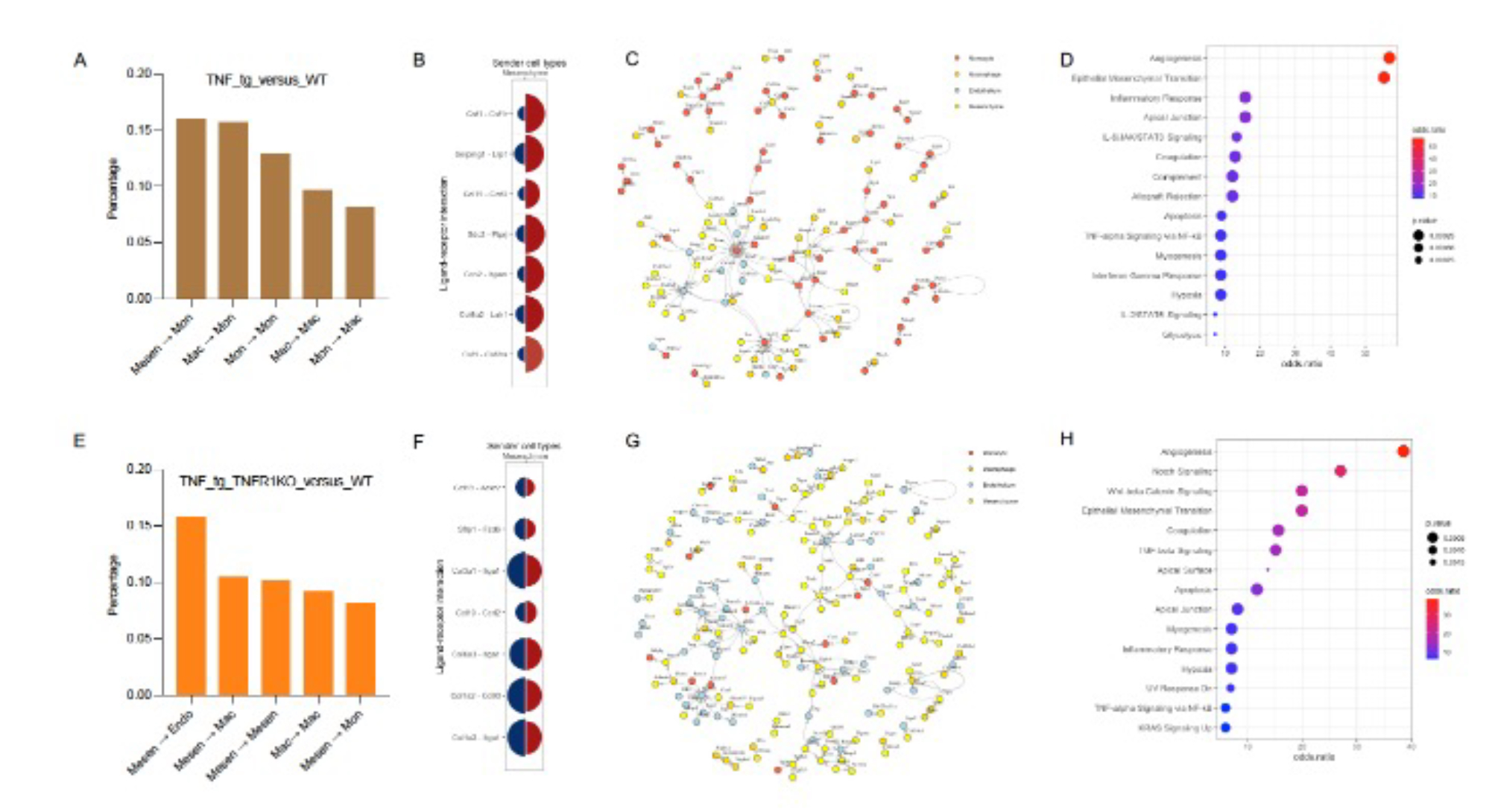
Results: Cells were clustered based on their expression profiles and cell types were annotated based on established cell markers from LungMap (Fig 1A). We observed dramatic expansion of myeloid cells and decreased cellular proportions of endothelial and mesenchymal cell in TNF-Tg and TNF-Tg-TNFR2KO mice as compared to WT and TNF-Tg/TNFR1KO mice (Fig 1B). We next focused on intercellular interaction among monocyte, macrophage, endothelial, and mesenchymal cells using NicheNet. When we compared signaling between 3 conditions (TNF-Tg, TNF-Tg/TNFR1KO, and TNF-Tg/TNFR2KO) and WT, we identified changes in global cellular communication with a preference for monocyte and macrophage interactions in the TNF-Tg, and TNF-Tg/TNFR2KO conditions (Fig 2A), while TNF-Tg/TNFR1KO mice retained interactions between mesenchymal cells and other cell types (Fig 2E). In all conditions, angiogenesis and epithelial mesenchymal transition were upregulated in the presence of TNF (Fig 2D/H). Monocytes and macrophages in the PH conditions mediated inflammatory processes including JAK/Stat, interferon gamma, complement, and TNF/NFkB (Fig 2C/D). Top ligand-receptor pairs between mesenchymal cells and monocytes include Csf1 with Csf1r, Ccl19 with Ccrl2, and Serping11 with Lrp1 (Fig 2B). Mesenchymal cell-cell interactions in TNF-Tg/TNFR1KO included Notch, Wnt/B-catenin, and TGF-beta pathways which were not seen in the PH conditions (Fig 2G/H). Top ligand-receptor pairs between mesenchymal and endothelial cells seen in this comparison include Ccl19 with Ackr2, Col1a2 with Cd93, and Sfrp1 with Fzd6.
Conclusion: Our results suggest that TNF-Tg mice develop PH promoted by an increase in myeloid cell infiltrate and corresponding TNF-TNFR1-mediated signaling pathways. Myeloid cell-cell interactions promote a variety of cellular changes including up-regulation of pro-inflammatory pathways in other cell types, and loss of homeostatic signaling mediated by mesenchymal cells.
To cite this abstract in AMA style:
Wang G, Duemmel S, Korman B. Ligand-Receptor Analysis Reveals Myeloid-Mediated Pro-Inflammatory Responses and Loss of Mesenchymal Signaling Driven by TNFR1 in Experimental Pulmonary Hypertension [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/ligand-receptor-analysis-reveals-myeloid-mediated-pro-inflammatory-responses-and-loss-of-mesenchymal-signaling-driven-by-tnfr1-in-experimental-pulmonary-hypertension/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/ligand-receptor-analysis-reveals-myeloid-mediated-pro-inflammatory-responses-and-loss-of-mesenchymal-signaling-driven-by-tnfr1-in-experimental-pulmonary-hypertension/