Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: A randomized, double-blind,
placebo-controlled, Phase III clinical trial showed that lesinurad, a selective
uric acid reabsorption inhibitor (SURI), in combination with febuxostat 80 mg
(FBX) significantly increased the proportion of patients with tophaceous gout who achieved the serum uric acid (sUA)
target of <5.0 mg/dL at 6 months compared to FBX alone. Lesinurad was
generally well tolerated, with the 200 mg dose having a safety profile comparable
to FBX alone, with the exception of a higher incidence in predominately
reversible serum creatinine (sCr) elevations. As renal impairment and
hyperuricemia frequently coexist, we aimed to analyze treatment with respect to
patient baseline renal function.
Methods: Patient data was obtained from the CRYSTAL study (NCT01510769)
where patients with estimated creatinine clearance (eCrCl; Cockcroft-Gault
formula using ideal body weight) <30 mL/min were excluded. In current
analyses, patients were analyzed by renal baseline function using eCrCl groups
of <60, <90, and ≥90 mL/min; statistical analyses were unadjusted
for multiplicity.
Results: In total, 324 patients were randomized and included in
these analyses. Demographic characteristics,
including age, gender, race, weight, and BMI, were broadly similar between
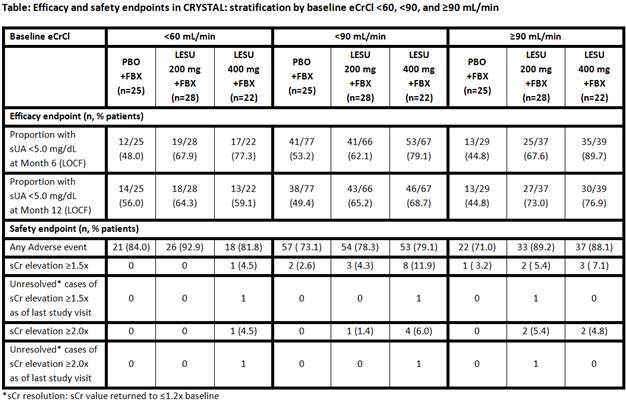
patient groups stratified by baseline renal function. The proportion of patients achieving sUA target levels of
<5.0 mg/dL was greater in the lesinurad 200 mg + FBX group compared with FBX
alone at 12 months in those with eCrCL <90 and ≥90
mL/min (P<0.05 for both) (Table).
For lesinurad 400 mg + FBX, the proportion of patients achieving <5.0 mg/dL
was greater than FBX alone at Month 6 (P<0.05 for <60; P<0.001 for
<90 and ≥90) and Month 12 (P<0.05 for <90 and ≥90).No
consistent differences in treatment-emergent AE rates were observed based on
baseline renal function (Table). sCr
elevations occurred at increased rates in the lesinurad groups (particularly
the 400 mg dose) versus FBX + placebo, without evident differences according to
baseline renal function.
Conclusion: The results of this Phase III study
subanalysis indicate that lesinurad in combination with febuxostat is
efficacious in all renal function groups. Safety findings were consistent
between treatment groups across all renal function categories.
To cite this abstract in AMA style:
Dalbeth N, Jones G, Terkeltaub R, Khanna D, Kopicko J, Adler S, Bhakta N, Fung M, Storgard C, Baumgartner S, Perez-Ruiz F. Lesinurad and Febuxostat Combination Therapy: Analysis of Treatment Based on Patient Baseline Renal Function [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/lesinurad-and-febuxostat-combination-therapy-analysis-of-treatment-based-on-patient-baseline-renal-function/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/lesinurad-and-febuxostat-combination-therapy-analysis-of-treatment-based-on-patient-baseline-renal-function/