Session Information
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Lesinurad (LESU; RDEA594)
is a selective uric acid reabsorption inhibitor (SURI) being investigated for
the treatment of gout in combination with a xanthine oxidase inhibitor. The CRYSTAL study is a
multinational, randomized, double-blind, placebo-controlled, Phase III clinical
trial of LESU in combination with febuxostat (FBX) to determine efficacy and
safety of combination therapy compared with FBX monotherapy in patients with
tophaceous gout (NCT01510769).
Methods: Patients with gout, aged 18–85 yrs, with serum
uric acid (sUA) ≥8 mg/dL (≥6 mg/dL on urate lowering therapy) and
≥1 tophus were given FBX 80 mg qd for 3 weeks before randomization to
LESU (200 mg or 400 mg oral, qd) in combination with FBX or placebo (PBO) +
FBX. Primary endpoint was proportion of patients with sUA <5.0 mg/dL by
Month 6. Secondary endpoints included proportion of patients with complete
resolution of ≥1 tophus and percent reduction in tophus area by Month 12.
Safety assessments included adverse events and laboratory data.
Results: Patients (N=324) were white (79.9%) and male
(95.4%) with mean±SD age of 54.1±11.0 yrs and 14.7±10.9 yrs since gout
diagnosis. sUA was 8.7±1.6 mg/dL at screening and 5.3±1.6 mg/dL on FBX at
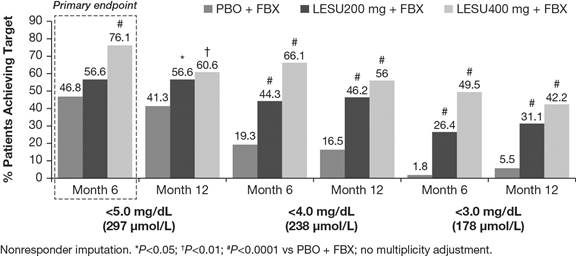
randomization (28% with sUA ≥6 mg/dL). More patients achieved sUA levels
<6, <5 (primary endpoint), <4, and <3 mg/dL with LESU+FBX (Figure).
Percentage of patients with complete resolution of ≥1 tophus by Month 12 was
21.1%, 25.5%, and 30.3% for FBX+PBO, FBX+LESU200, and FBX+LESU400,
respectively. Percent decrease in tophus area by Month 12 was 55.8% and 57.9%
for FBX+LESU200 and FBX+LESU400, respectively, vs 31.3% for FBX + PBO (both nominal
P<0.05). Safety data are reported in the Table. More serum creatinine
(sCr) increases were observed with LESU+FBX; most resolved by last study visit
(Table). Kidney stones were lower with LESU.
Conclusion: In patients with tophaceous
gout, LESU (200 or 400 mg) in combination with FBX increased the proportion of
patients achieving sUA <5 mg/dL at 6 months compared with FBX alone. LESU in
combination with FBX resulted in greater tophus area resolution compared with
FBX alone, as well as a dose-ordered numerical increase in the proportion of
subjects having complete tophi resolution. LESU was generally well tolerated,
except for higher incidence of predominately reversible sCr elevations.
Combination therapy with LESU+FBX may represent a future treatment option for
patients with tophaceous gout on FBX who warrant additional therapy.
|
|
PBO + FBX N=109 |
LESU200 + FBX N=106 |
LESU400 + FBX N=109 |
|
Patients experiencing any TEAE |
79 (72.5%) |
87 (82.1%) |
90 (82.6%) |
|
Patients with serious TEAEs |
10 (9.2%) |
6 (5.7%) |
9 (8.3%) |
|
Patients with any renal-related AEs |
6 (5.5%) |
9 (8.5%) |
11 (10.1%) |
|
Patients with serious renal-related AEs |
1 (0.9%) |
0 (0%) |
2 (1.8%) |
|
Patients with kidney stones |
4 (3.7%) |
1 (0.9%) |
2 (1.8%) |
|
Patients with ≥2.0x increase in sCr Number (%) sCr elevations resolved by last study visit |
0 (0%) – |
3 (2.8%) 2/3 (67%) |
6 (5.5%) 6/7 (86%) |
|
Data are n (%). |
|||
To cite this abstract in AMA style:
Dalbeth N, Jones G, Terkeltaub R, Khanna D, Kopicko J, Bhakta N, Fung M, Storgard C, Baumgartner S, Perez-Ruiz F. Lesinurad, a Novel Selective Uric Acid Reabsorption Inhibitor, in Combination with Febuxostat, in Patients with Tophaceous Gout [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/lesinurad-a-novel-selective-uric-acid-reabsorption-inhibitor-in-combination-with-febuxostat-in-patients-with-tophaceous-gout/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/lesinurad-a-novel-selective-uric-acid-reabsorption-inhibitor-in-combination-with-febuxostat-in-patients-with-tophaceous-gout/