Session Information
Date: Monday, November 8, 2021
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: SARS-CoV-2 infection can lead to severe inflammation including increased complement activation (Ma, Kulkarni 2021) and the production of several proinflammatory cytokines. The rapid deployment of novel, effective vaccines against SARS-CoV-2 allow for more in-patient visits across the medical landscape, but little is known about the short-term impact the SARS-CoV-2 vaccines may have on immunological testing. This study examined a mutlianalyte assay panel (MAP), including cell-bound complement activation products (CB-CAPs), as well as levels of cytokines in normal healthy volunteers before and shortly after SARS-CoV-2 vaccination, to explore any impact immunization may have on complement activation, canonical connective-tissue disease markers, or the cytokine milieu.
Methods: Normal healthy volunteers (n = 6; 3 females, 3 males) were tested before and within two weeks (2 subjects each 6-, 11-, and 14-days post-vaccination) of receiving their second SARS-CoV-2 vaccination (Moderna mRNA-1273). Serum C3 and C4 levels were measured using immunoturbidimetry and CB-CAPs were measured from EDTA blood using flow cytometry and expressed as net mean fluorescence intensity (MFI). Autoantibodies were measured by ELISA and anti-dsDNA positivity was confirmed by Crithidia luciliae immunofluorescence. Cytokines were measured in plasma or serum by electrochemiluminescence on the Meso Scale Discovery platform. F-tests were performed to describe variance, and p-values were determined by paired, two-tailed t-test.
Results: There were no significant differences between pre (4-22 months prior)- and post-vaccination in the levels of erythrocyte-bound C4d (EC4d), B-cell bound C4d (BC4d), anti-nuclear antibodies (ANA), C3, or C4, as measured by a 2-tailed equal variance t-test (Figure 1). Accounting for unequal variance found between populations (f-test = 0.02), anti-dsDNA also showed no significant differences pre- and post-vaccination (note: the one anti-dsDNA positive result was not Crithidia positive). The CB-CAPs remained well below the positivity threshold of 15 net MFI for EC4d and 61 net MFI for BC4d, regardless of vaccination status (Figure 1). Additionally, levels of inflammatory biomarkers were similar pre- and post-vaccination (Table 1).
Conclusion: Although COVID-19 induces robust inflammation and complement activation, SARS-CoV-2 vaccination does not appear to affect levels of serum complement, complement activation, or cytokine milieu in the 2 weeks post-vaccination. In particular, as erythrocytes circulate for up to 90 days, EC4d can be measured for up to 3 months. If robust complement activation were to occur following vaccination, one might expect increased EC4d in those receiving the SARS-CoV-2 vaccine, thus distorting its diagnostic value. Notably, CB-CAPs positivity rate has remained stable among routinely ordered testing pre-pandemic and post-vaccine rollout time periods (Figure 2). Together, these findings should increase the confidence of any physician ordering a diagnostic MAP containing CB-CAPs even in patients who received the SARS-CoV-2 vaccine.
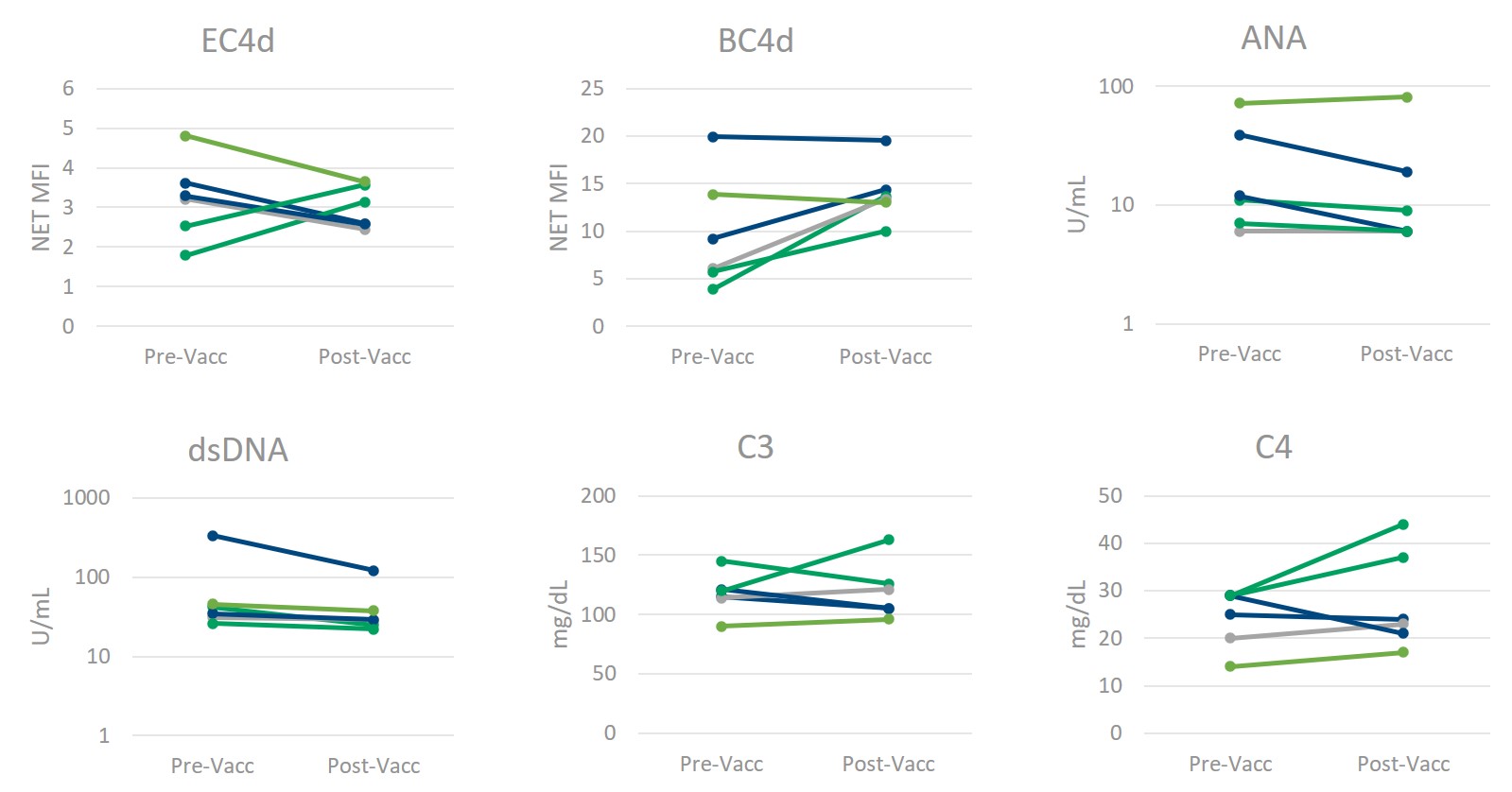 Figure 1) Multianalyte Assay Panel (MAP) components remain constant following SARS-CoV_2 Vaccination. The levels of erythrocyte- and B-cell bound C4d (measured by flow cytometry, reported as Net MFI), anti-nuclear antibodies (ANA) and anti-dsDNA (measured by ELISA, reported in Units/mL), and complement proteins C3 and C4 (measured by immunoturbidimetry, reported as mg/dL) do not significantly change in normal healthy individuals following SARS-CoV_2 vaccination. (Confirmed by paired, two-tailed homoscedastic t-test for all but anti-dsDNA (heteroscedastic).)
Figure 1) Multianalyte Assay Panel (MAP) components remain constant following SARS-CoV_2 Vaccination. The levels of erythrocyte- and B-cell bound C4d (measured by flow cytometry, reported as Net MFI), anti-nuclear antibodies (ANA) and anti-dsDNA (measured by ELISA, reported in Units/mL), and complement proteins C3 and C4 (measured by immunoturbidimetry, reported as mg/dL) do not significantly change in normal healthy individuals following SARS-CoV_2 vaccination. (Confirmed by paired, two-tailed homoscedastic t-test for all but anti-dsDNA (heteroscedastic).)
 Table 1) Normal healthy median and interquartile range values pre- and post-SARS-CoV_2 vaccination. Results of EC4d and BC4d (Net MFI), ANA and anti-dsDNA (U/mL), C3 and C4 concentration (mg/dL), and cytokine concentrations (interferon-gamma (IFNγ), interleukins (IL)-6, -8, _10, _17A, and 18, tumor necrosis factor-alpha (TNFα), IFNγ-induced protein_10 (IP_10), interferon-alpha_2a (IFN-α2a), and monocyte chemoattractant protein_1 (MCP_1) (pg/mL) in normal healthy volunteers prior to and following SARS-CoV_2 vaccination. Multianalyte Assay Panel (MAP) score is calculated by a combination of CB-CAP and auto-antibody test results, and where a score below 0 is determined to be a negative result.
Table 1) Normal healthy median and interquartile range values pre- and post-SARS-CoV_2 vaccination. Results of EC4d and BC4d (Net MFI), ANA and anti-dsDNA (U/mL), C3 and C4 concentration (mg/dL), and cytokine concentrations (interferon-gamma (IFNγ), interleukins (IL)-6, -8, _10, _17A, and 18, tumor necrosis factor-alpha (TNFα), IFNγ-induced protein_10 (IP_10), interferon-alpha_2a (IFN-α2a), and monocyte chemoattractant protein_1 (MCP_1) (pg/mL) in normal healthy volunteers prior to and following SARS-CoV_2 vaccination. Multianalyte Assay Panel (MAP) score is calculated by a combination of CB-CAP and auto-antibody test results, and where a score below 0 is determined to be a negative result.
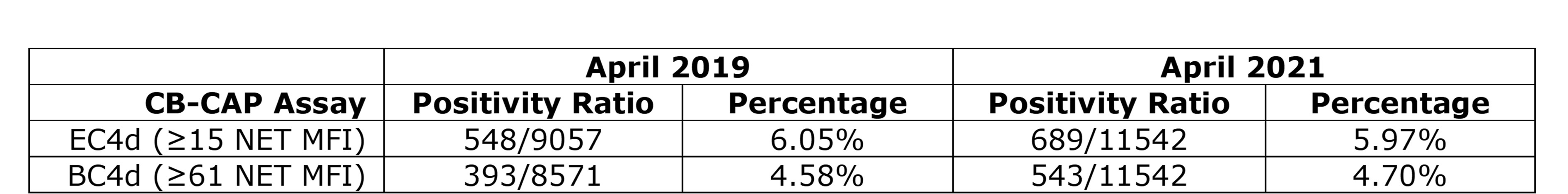 Table 2) EC4d and BC4d positivity rates in April 2019 versus April 2021. Positivity of EC4d (≥ 15 NET MFI) and BC4d (≥ 61 NET MFI) among clinical samples run prior to the SARS-CoV_2 pandemic (April 2019) and amid the public rollout of SARS-CoV_2 vaccines (April 2021).
Table 2) EC4d and BC4d positivity rates in April 2019 versus April 2021. Positivity of EC4d (≥ 15 NET MFI) and BC4d (≥ 61 NET MFI) among clinical samples run prior to the SARS-CoV_2 pandemic (April 2019) and amid the public rollout of SARS-CoV_2 vaccines (April 2021).
To cite this abstract in AMA style:
Rudolph M, Kammensheidt A, Alexander R. Lack of Effect of SARS-CoV-2 Vaccination on Cell Bound Complement Activation Products (CB-CAPs), Multianalyte Assay Panel (MAP) with Algorithm, and Inflammatory Biomarkers [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/lack-of-effect-of-sars-cov-2-vaccination-on-cell-bound-complement-activation-products-cb-caps-multianalyte-assay-panel-map-with-algorithm-and-inflammatory-biomarkers/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/lack-of-effect-of-sars-cov-2-vaccination-on-cell-bound-complement-activation-products-cb-caps-multianalyte-assay-panel-map-with-algorithm-and-inflammatory-biomarkers/
