Session Information
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Tanezumab, a monoclonal antibody against nerve growth factor, is in development for the relief of signs and symptoms of moderate to severe osteoarthritis (OA) in adult patients (pts) for whom use of other analgesics is ineffective or not appropriate. Previously, tanezumab has been associated with an increased incidence of joint safety events versus nonsteroidal anti-inflammatory drugs (NSAIDs)1. Our aim was to investigate the benefit/harm relationship for tanezumab and NSAIDs using the OMERACT 3×3 scale2.
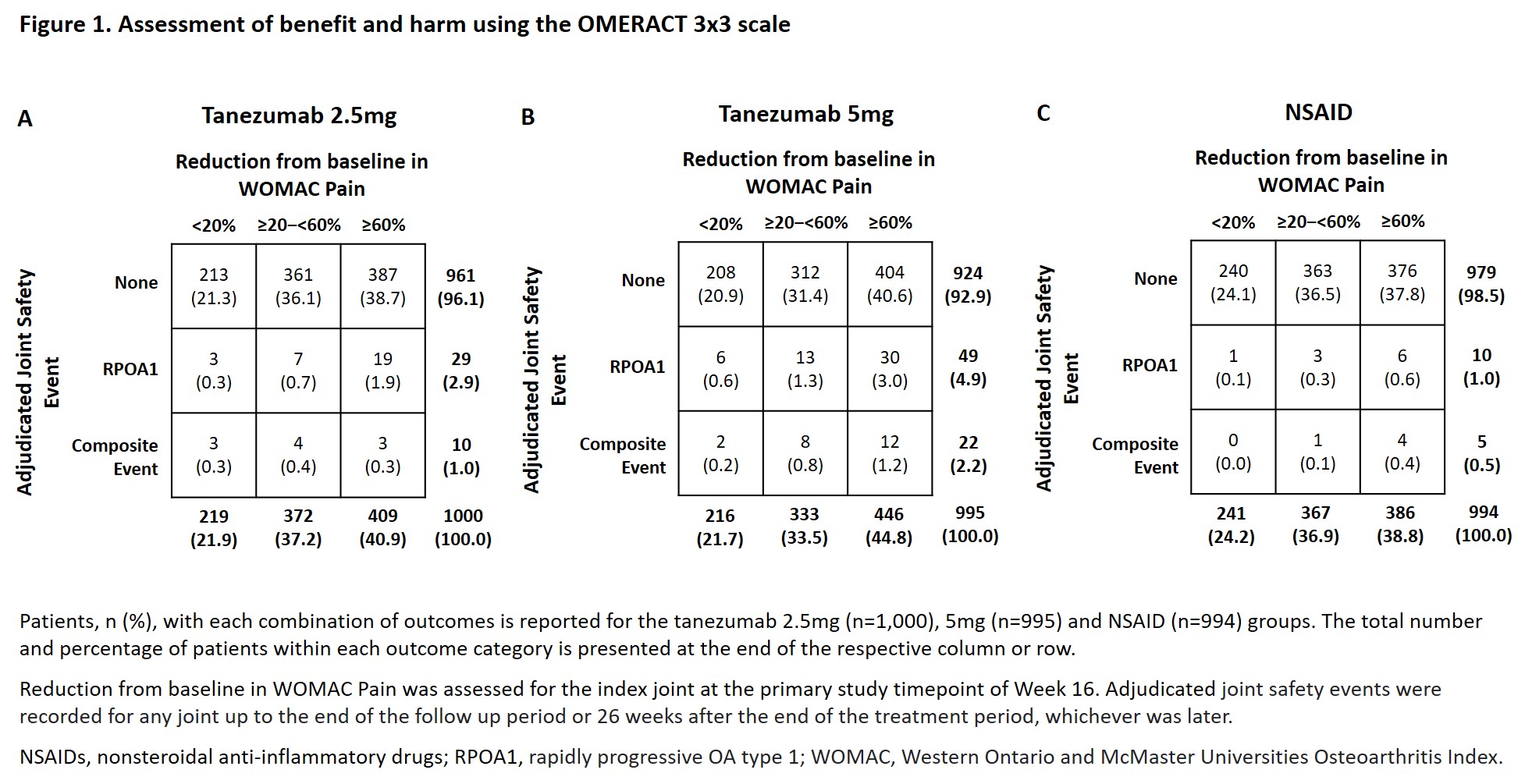
Methods: The study (NCT02528188) included pts with moderate to severe hip or knee OA with a history of inadequate pain relief with acetaminophen; inadequate pain relief with/intolerance to tramadol or opioids; or unwillingness to take opioids. Pts were randomized to subcutaneous tanezumab 2.5mg or 5mg every 8 weeks or twice daily oral NSAIDs for a total of 56 weeks, with a 24-week post-treatment follow-up period. Benefit was assessed using 3 categories of reduction from baseline in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC)* Pain in the index joint at the primary efficacy endpoint of Week 16; < 20% (minimal improvement), ≥20–< 60% (moderate–substantial improvement) or ≥60% (very substantial improvement). Harm was assessed by considering 3 categories of joint safety outcomes; none (no adjudicated joint safety endpoints), rapidly progressive OA type 1 (RPOA1) or having an adjudicated composite joint safety endpoint (primary osteonecrosis, RPOA type 2, subchondral insufficiency fracture or pathological fracture) in any joint up to the end of the follow-up period or 26 weeks after the end of the treatment period, whichever was later.
*© 1996 Nicholas Bellamy. WOMAC® is a registered trademark of Nicholas Bellamy (CDN, EU, USA).
Results: In the tanezumab 2.5mg group (n=1,000), 387 pts (38.7%) had the most favorable outcome of ≥60% improvement in WOMAC Pain with no joint safety events. 3 pts (0.3%) had the least favorable outcome of < 20% improvement in WOMAC Pain and a composite event (Figure 1A). In the tanezumab 5mg group (n=995), 404 pts (40.6%) had the most favorable outcome and 2 pts (0.2%) had the least favorable outcome (Figure 1B). In the NSAID group (n=994), 376 pts (37.8%) had the most favorable outcome and 0 pts had the least favorable outcome (Figure 1C). Across all treatment groups, the incidence of RPOA1 increased with the category of benefit. The number of pts meeting the composite event increased with the category of benefit in the tanezumab 5mg and NSAID groups.
Conclusion: These analyses showed that ≥37.8% of pts had the most favorable outcome and ≤0.3% had the least favorable outcome; the proportion of pts with these outcomes was not notably different across treatments. An increased incidence of adjudicated joint safety events was associated with more favorable efficacy responses across treatments, with the exception of the composite event in the tanezumab 2.5mg group.
This study was sponsored by Pfizer and Eli Lilly. Medical writing support was provided by Steven Moore, PhD, of Engage Scientific Solutions and was funded by Pfizer and Eli Lilly.
References
1Hochberg MC, et al. Arthritis Rheumatol 2021; DOI 10.1002/art.41674
2Boers M, et al. J. Clin. Epidemiol 2010; 63: 627-32
To cite this abstract in AMA style:
Fountaine R, Dworkin R, Hickman A, Pixton G, Whalen E, West C, Verburg K. Joint Safety of Tanezumab versus NSAIDs; A Combined Assessment of Benefit and Harm [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/joint-safety-of-tanezumab-versus-nsaids-a-combined-assessment-of-benefit-and-harm/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/joint-safety-of-tanezumab-versus-nsaids-a-combined-assessment-of-benefit-and-harm/