Session Information
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Lymphatic dysfunction is a factor in the onset and progression of rheumatoid arthritis (RA) (1). Our prior studies demonstrated reduced lymphatic clearance in the hands of symptomatic RA patients vs. healthy controls (2). We reported similar findings in the tumor necrosis factor transgenic (TNF-Tg) mouse model of RA, which exhibits reduced contractility of joint-draining popliteal lymphatic vessels (PLVs) (3,4). Remarkably, anti-TNF therapy in flaring TNF-Tg mice recovers PLV contractions concomitant with amelioration of arthritis (4). Based on this result, we hypothesize that PLV dysfunction correlates with arthritis and is caused by: 1) chronic TNF-mediated lymphatic muscle cell (LMC) damage (4), and/or 2) paracrine factors known to inhibit lymphatic contractions from accumulated peri-lymphatic inflammatory cells during chronic inflammation (5). To specifically assess LMC tissue damage, herein we tested the hypotheses that: 1) alpha-smooth muscle actin (αSMA)+ PLV-LMC coverage is reduced in TNF-Tg mice with severe arthritis, and 2) anti-TNF therapy recovers LMC integrity by promoting turnover of bromodeoxyuridine (BrdU)+ PLV-LMCs associated with return of lymphatic and joint homeostasis.
Methods: BrdU (0.1mg/g/day/i.p.) was administered to 8-month-old wild-type (WT, n=8 mice) and TNF-Tg (placebo or anti-TNF therapy, n=5 mice each; i.p. 10mg/kg/week) mice for 6-consecutive weeks, followed by whole mount immunofluorescent microscopy for αSMA+ and BrdU+ PLV-LMCs, as previously described (6). Talus bone volumes were measured by μCT longitudinally every 3-weeks as a biomarker of ankle arthritis. Preliminary scanning electron microscopy (SEM) was performed on WT PLVs (n=2 mice) to assess the feasibility of evaluating LMC coverage with ultrastructural analysis.
Results: αSMA+ PLV-LMC coverage was reduced in TNF-Tg placebo mice vs. WT littermates (61.32±23.47% placebo vs 84.99±10.79% WT; p< 0.0001). However, anti-TNF therapy failed to recover αSMA+ PLV-LMC coverage (68.86±20.41%) (Figure 1), consistent with no change in LMC turnover (< 2% BrdU+/αSMA+ cells for all groups) (Figure 2). In contrast, anti-TNF therapy restored talus bone volume, and there was no relationship with αSMA+ PLV-LMC coverage and bone volume (R2=0.1106) (Figure 1). SEM of PLVs confirmed the ability to further interrogate LMC coverage with ultrastructural imaging, and also unexpectedly identified a predominant peri-lymphatic mast cell population that may explain previous findings of increased peri-lymphatic cells in TNF-Tg mice (Figure 3).
Conclusion: Chronic inflammation reduces αSMA+ PLV-LMC coverage. While 6-weeks of anti-TNF therapy reverses bone loss in TNF-Tg mice, this short-course does not enhance αSMA investiture or LMC turnover in damaged PLVs. Thus, future work will focus on the recent discovery of mast cell accumulation in the peri-lymphatic tissue of joint-draining PLVs during chronic inflammation, known to inhibit LMC contractility.
1. Bouta et al. Nat Rev Rheum. 14(2):94-106. 2018.
2. Bell et al. A&R. 72(9):1447-1455. 2020.
3. Scallan et al. BioRxiv. 2020.
4. Bouta et al. A&R. 69(6):1187-1193. 2017.
5. Li et al. A&R. 65(1):130-138. 2013.
6. Kenney et al. Sci Rep. 10(1):18088. 2020.
 Figure 1. 6-weeks of anti-TNF therapy ameliorates bone erosions despite persistence of reduced PLV-LMC αSMA coverage in TNF-Tg mice. TNF-Tg mice (8-month-old) were treated with anti-TNF or placebo (10mg/kg/week, i.p.) for 6-consecutive weeks, while WT littermates received volume-matched vehicle PBS. Following treatment, PLVs were harvested and processed for whole mount immunofluorescent microscopy. Talus bone volumes were evaluated longitudinally at 3-week intervals by μCT. Representative images of αSMA+ PLV-LMC coverage (green) and segmented taluses (colored bone within the remaining transparent grey ankle joint) (WT A-C; TNF-Tg D-F; anti-TNF G-I) with quantification of αSMA coverage (J, αSMA coverage as a percent of total PLV area), talus bone volumes (K), and the correlation between these outcomes (L) are shown for all groups. Note the similar reduction in intensity of αSMA signal for both placebo and anti-TNF treated TNF-Tg cohorts despite talus bone recovery with anti-TNF therapy. Each data point for αSMA coverage represents individual PLVs, while correlation with talus bone volumes was performed with the average between the two PLVs present in each limb. Statistics: All data is reported as mean ± standard deviation (SD). One-Way ANOVA (J; **** p < 0.0001, ** p < 0.01); Two-Way ANOVA (K; **** p < 0.0001, *** p < 0.001); and linear regression (L; no significance (ns) p > 0.05).
Figure 1. 6-weeks of anti-TNF therapy ameliorates bone erosions despite persistence of reduced PLV-LMC αSMA coverage in TNF-Tg mice. TNF-Tg mice (8-month-old) were treated with anti-TNF or placebo (10mg/kg/week, i.p.) for 6-consecutive weeks, while WT littermates received volume-matched vehicle PBS. Following treatment, PLVs were harvested and processed for whole mount immunofluorescent microscopy. Talus bone volumes were evaluated longitudinally at 3-week intervals by μCT. Representative images of αSMA+ PLV-LMC coverage (green) and segmented taluses (colored bone within the remaining transparent grey ankle joint) (WT A-C; TNF-Tg D-F; anti-TNF G-I) with quantification of αSMA coverage (J, αSMA coverage as a percent of total PLV area), talus bone volumes (K), and the correlation between these outcomes (L) are shown for all groups. Note the similar reduction in intensity of αSMA signal for both placebo and anti-TNF treated TNF-Tg cohorts despite talus bone recovery with anti-TNF therapy. Each data point for αSMA coverage represents individual PLVs, while correlation with talus bone volumes was performed with the average between the two PLVs present in each limb. Statistics: All data is reported as mean ± standard deviation (SD). One-Way ANOVA (J; **** p < 0.0001, ** p < 0.01); Two-Way ANOVA (K; **** p < 0.0001, *** p < 0.001); and linear regression (L; no significance (ns) p > 0.05).
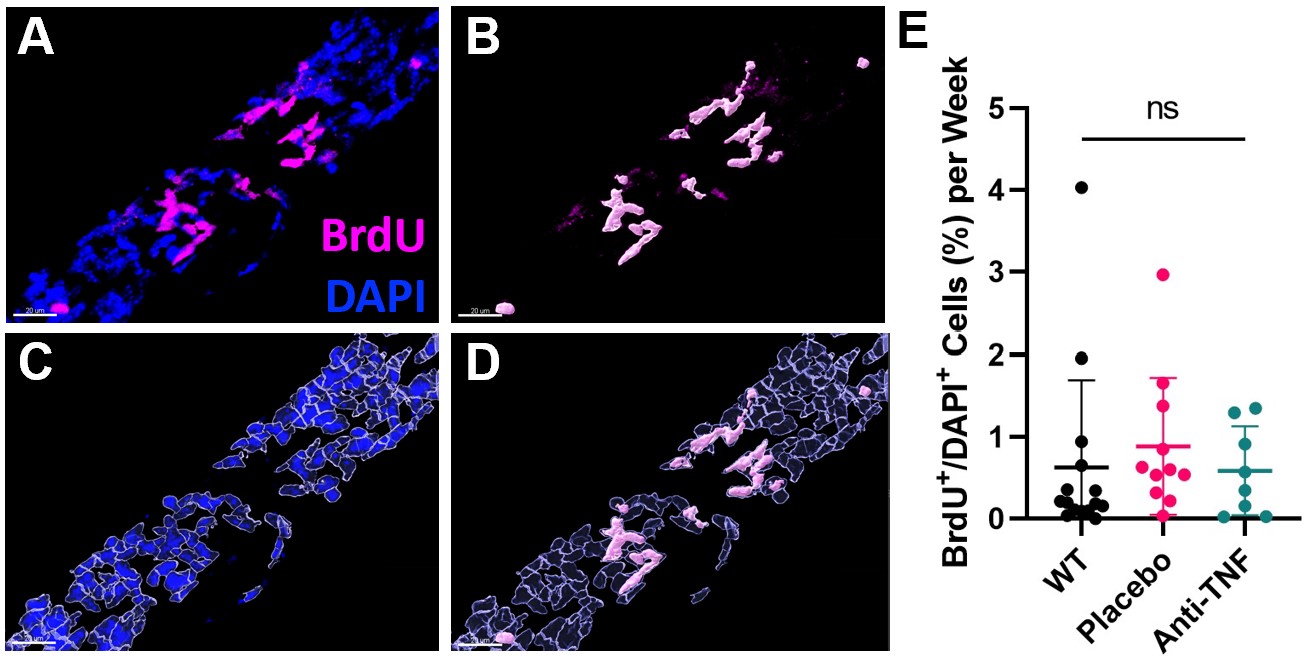 Figure 2. LMC turnover of joint-draining PLVs is unchanged during inflammation or therapy. Imaris software was used to analyze DAPI+/BrdU+ cell colocalization within an αSMA+ mask in 200x confocal stacks across the entire length of PLVs (~5mm) (A). Segmentation of individual BrdU+ (B) and DAPI+ nuclei (C) was performed to evaluate the ratio of BrdU+/DAPI+ cells (D). WT and TNF-Tg (placebo or anti-TNF as in Figure 1) were treated with BrdU for 6-consecutive weeks (0.1mg/g/day, i.p., as previously described (6)), and quantification of BrdU labeled cells in the PLVs indicates no difference in PLV cellular turnover between groups (E). Each data point represents a single PLV. Statistics: All data is reported as mean ± SD. One-Way ANOVA (E; ns p > 0.05 all comparisons).
Figure 2. LMC turnover of joint-draining PLVs is unchanged during inflammation or therapy. Imaris software was used to analyze DAPI+/BrdU+ cell colocalization within an αSMA+ mask in 200x confocal stacks across the entire length of PLVs (~5mm) (A). Segmentation of individual BrdU+ (B) and DAPI+ nuclei (C) was performed to evaluate the ratio of BrdU+/DAPI+ cells (D). WT and TNF-Tg (placebo or anti-TNF as in Figure 1) were treated with BrdU for 6-consecutive weeks (0.1mg/g/day, i.p., as previously described (6)), and quantification of BrdU labeled cells in the PLVs indicates no difference in PLV cellular turnover between groups (E). Each data point represents a single PLV. Statistics: All data is reported as mean ± SD. One-Way ANOVA (E; ns p > 0.05 all comparisons).
 Figure 3. Increased mast cell numbers around PLVs in TNF-Tg mice with inflammatory arthritis. PLVs harvested from TNF-Tg mice exhibited increased cell accumulation in the peri-lymphatic region compared to those of WT controls by whole mount immunofluorescent microscopy (A,B), and the number of positive cells is quantified for each PLV (C). To elucidate the identity of these cells, scanning electron microscopy (SEM) was performed on WT PLVs, which identified LMCs (1,000x) (D) with mast cells embedded within the peri-lymphatic tissue (500x) (E), which was validated by histologic staining using toluidine blue (F). These findings suggest that during chronic inflammation, mast cells accumulate in the peri-lymphatic region surrounding joint-draining PLVs and inhibit lymphatic contractility associated with progression of arthritis in the afferent ankle. Future studies will target these identified mast cells (5,000x) (G) to further elucidate the mechanisms of lymphatic dysfunction in inflammatory arthritis. Statistics: All data is reported as mean ± SD. Unpaired t-test (C; * p < 0.05).
Figure 3. Increased mast cell numbers around PLVs in TNF-Tg mice with inflammatory arthritis. PLVs harvested from TNF-Tg mice exhibited increased cell accumulation in the peri-lymphatic region compared to those of WT controls by whole mount immunofluorescent microscopy (A,B), and the number of positive cells is quantified for each PLV (C). To elucidate the identity of these cells, scanning electron microscopy (SEM) was performed on WT PLVs, which identified LMCs (1,000x) (D) with mast cells embedded within the peri-lymphatic tissue (500x) (E), which was validated by histologic staining using toluidine blue (F). These findings suggest that during chronic inflammation, mast cells accumulate in the peri-lymphatic region surrounding joint-draining PLVs and inhibit lymphatic contractility associated with progression of arthritis in the afferent ankle. Future studies will target these identified mast cells (5,000x) (G) to further elucidate the mechanisms of lymphatic dysfunction in inflammatory arthritis. Statistics: All data is reported as mean ± SD. Unpaired t-test (C; * p < 0.05).
To cite this abstract in AMA style:
Kenney H, Peng Y, de Mesy Bentley K, Galloway C, Rahimi H, Xing L, Ritchlin C, Schwarz E. Joint-Draining Popliteal Lymphatic Vessels Exhibit Lymphatic Muscle Cell Dysfunction in TNF-Tg Mice with Inflammatory Arthritis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/joint-draining-popliteal-lymphatic-vessels-exhibit-lymphatic-muscle-cell-dysfunction-in-tnf-tg-mice-with-inflammatory-arthritis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/joint-draining-popliteal-lymphatic-vessels-exhibit-lymphatic-muscle-cell-dysfunction-in-tnf-tg-mice-with-inflammatory-arthritis/
