Session Information
Date: Sunday, November 8, 2020
Title: B Cell Biology & Targets in Autoimmune & Inflammatory Disease Poster
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Idiopathic inflammatory myopathies (IIM) are systemic autoimmune diseases traditionally classified as dermatomyositis or polymyositis, but these disorders are increasingly defined by the presence of specific autoantibodies. Anti-Jo-1 autoantibodies recognize histidyl tRNA synthetase and are found in a subset of these patients, but anti-Jo-1 B cells have not been previously characterized. To target these antigen-presenting cells in anti-histidyl-tRNA synthetase syndrome (HARS), we must first understand how they expand in the repertoire. In this study, we aimed to characterize Jo-1-binding B cells in HARS.
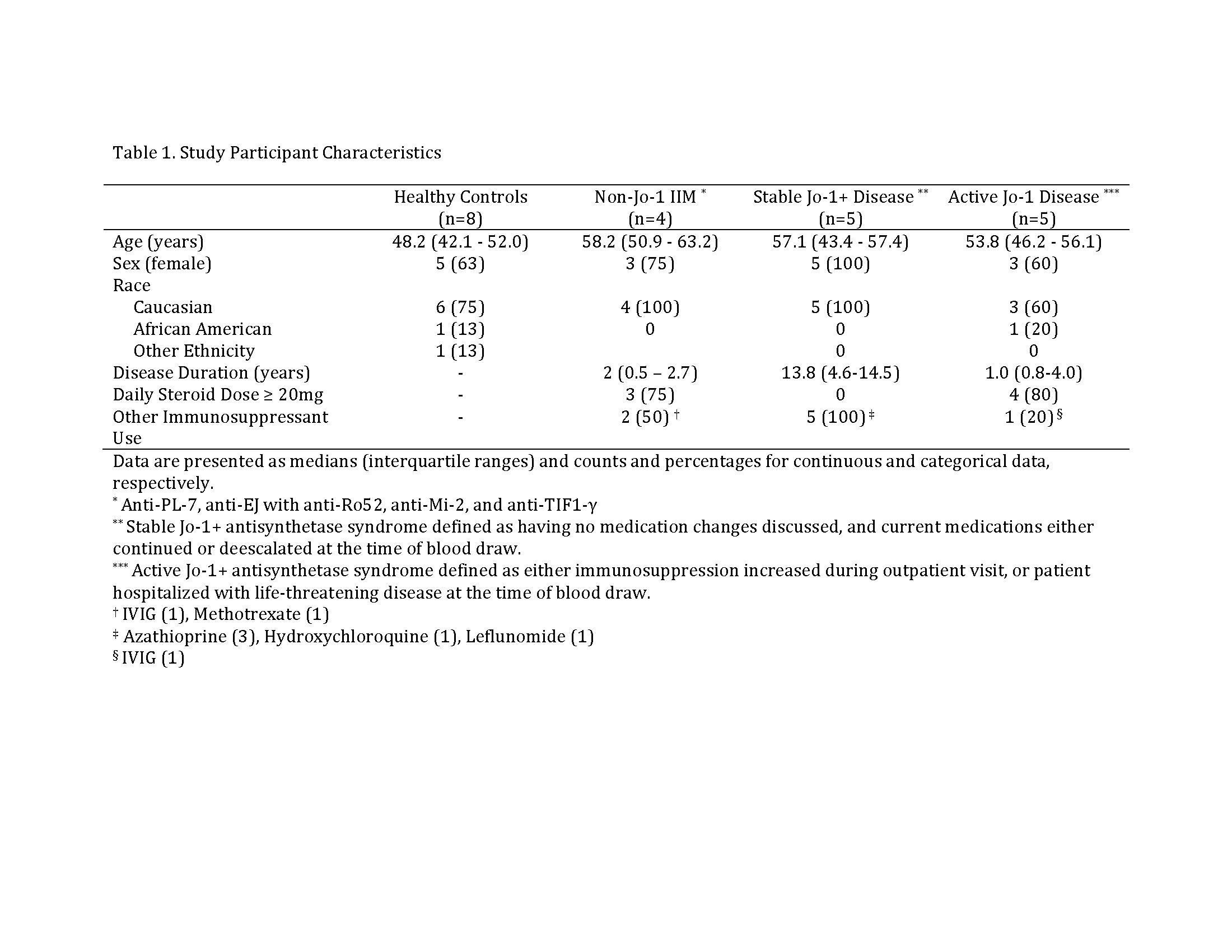
Methods: We enrolled 10 patients diagnosed with HARS, 4 patients with non-Jo-1 IIM, and 8 age and sex-matched healthy controls. We performed flow cytometry to phenotype peripheral blood mononuclear cells (PBMCs) and define the B cell subsets in which Jo-1-binding B cells reside. We polyclonally stimulated PBMCs from HARS subjects to drive in vitro B cell differentiation into antibody-secreting cells, and phenotypically compared Jo-1-binding versus non-Jo-1 binding B cells from the same subjects.
Results: On average, 65% of Jo-1-binding B cells were IgM+ (not class-switched). Compared to other B cells from the same donor, anti-Jo-1 B cells contained a higher percentage of autoimmune-prone CD21lo cells, increased CD38+CD27+ memory cells, but fewer CD38hiCD24– plasmablasts. Consistent with phenotypic data, anti-Jo-1 B cells were less likely to differentiate into CD38hi24– plasmablasts following in vitro stimulation.
Conclusion: Jo-1-binding B cells were identified in the peripheral blood of all HARS patients assessed, suggesting they can be tracked as biomarkers to assess whether experimental therapies limit their expansion and activation. These data suggest complex B cell biology exists beyond class-switched cells that differentiate to secrete anti-Jo-1 autoantibody, and provide an important advance towards defining antigen-specific B cells and developing novel B cell-directed therapies for HARS patients.
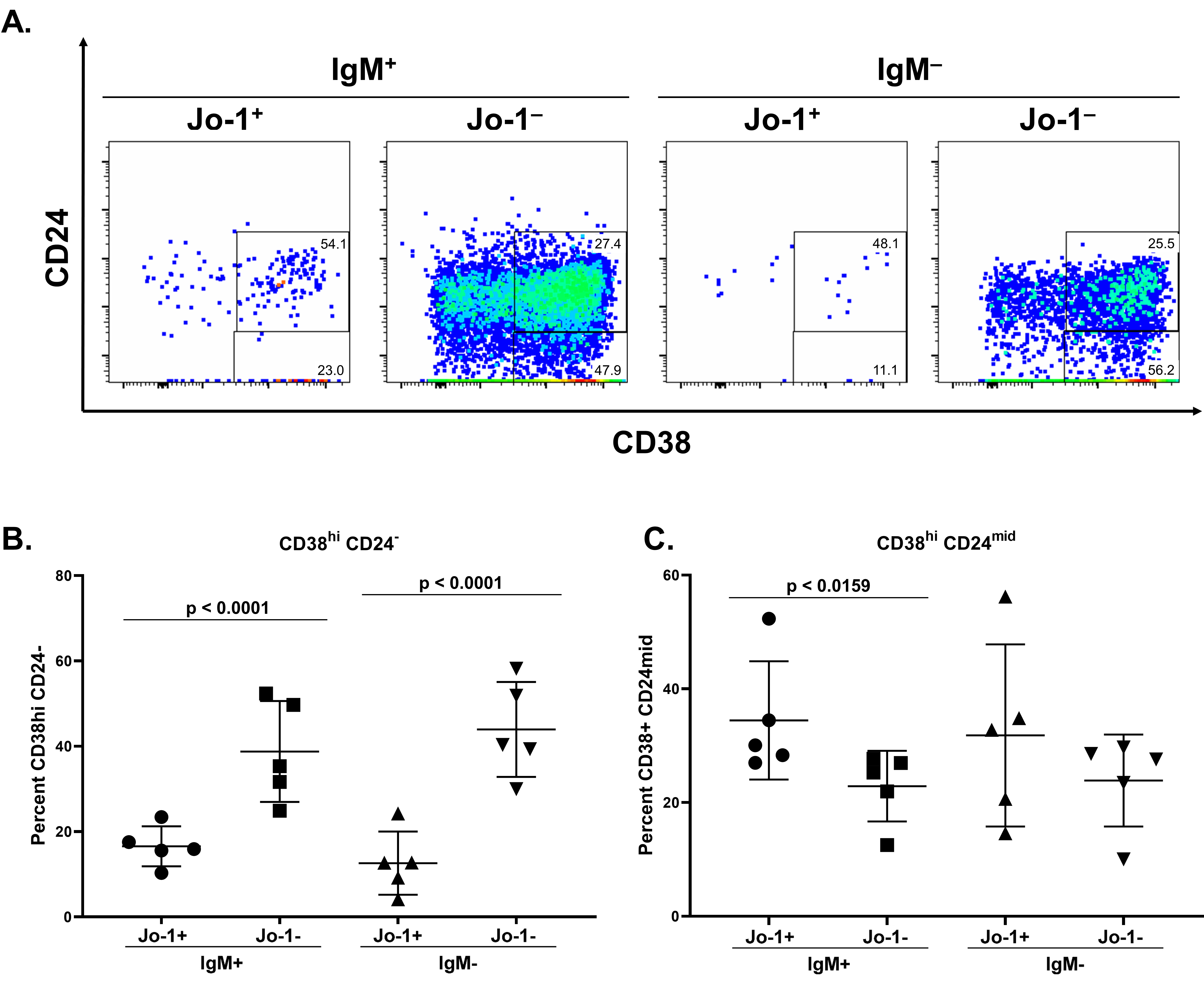 A lower frequency of Jo-1-binding B cells acquire a plasmablast phenotype following stimulation in vitro compared to non-Jo-1-binding B cells from the same IIM patients. PBMCs from n=5 HARS patients with active disease were polyclonally stimulated as in Methods. Cultured cells from 40-60 wells per patient were harvested on day 6 and stained for flow cytometry analysis. A) CD24 and CD38 expression is shown in representative flow plots derived from a single well of stimulated PBMCs. Comparison of relative frequencies for B) CD38 hi CD24 – B lymphocytes or C) CD38 + CD24 mid B lymphocytes between Jo-1 specific and Jo- 1 non-specific populations. Significant differences between IgM + Jo-1 + and IgM + Jo-1 – or IgM – Jo- 1 + and IgM – Jo-1 – populations as calculated using Mann-Whitney U test are indicated. The data are represented as the mean for each population within a given stimulation, the bars indicate the mean ± SD.
A lower frequency of Jo-1-binding B cells acquire a plasmablast phenotype following stimulation in vitro compared to non-Jo-1-binding B cells from the same IIM patients. PBMCs from n=5 HARS patients with active disease were polyclonally stimulated as in Methods. Cultured cells from 40-60 wells per patient were harvested on day 6 and stained for flow cytometry analysis. A) CD24 and CD38 expression is shown in representative flow plots derived from a single well of stimulated PBMCs. Comparison of relative frequencies for B) CD38 hi CD24 – B lymphocytes or C) CD38 + CD24 mid B lymphocytes between Jo-1 specific and Jo- 1 non-specific populations. Significant differences between IgM + Jo-1 + and IgM + Jo-1 – or IgM – Jo- 1 + and IgM – Jo-1 – populations as calculated using Mann-Whitney U test are indicated. The data are represented as the mean for each population within a given stimulation, the bars indicate the mean ± SD.
To cite this abstract in AMA style:
Young-Glazer J, Cisneros A, Wilfong E, Smith S, Crofford L, Bonami R. Jo-1-Binding B Cells Undergo Limited Class-Switching but Are Biased Towards Autoreactive-Prone and Memory B Cell Subsets in Anti-histidyl-tRNA Synthetase Syndrome [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/jo-1-binding-b-cells-undergo-limited-class-switching-but-are-biased-towards-autoreactive-prone-and-memory-b-cell-subsets-in-anti-histidyl-trna-synthetase-syndrome/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/jo-1-binding-b-cells-undergo-limited-class-switching-but-are-biased-towards-autoreactive-prone-and-memory-b-cell-subsets-in-anti-histidyl-trna-synthetase-syndrome/