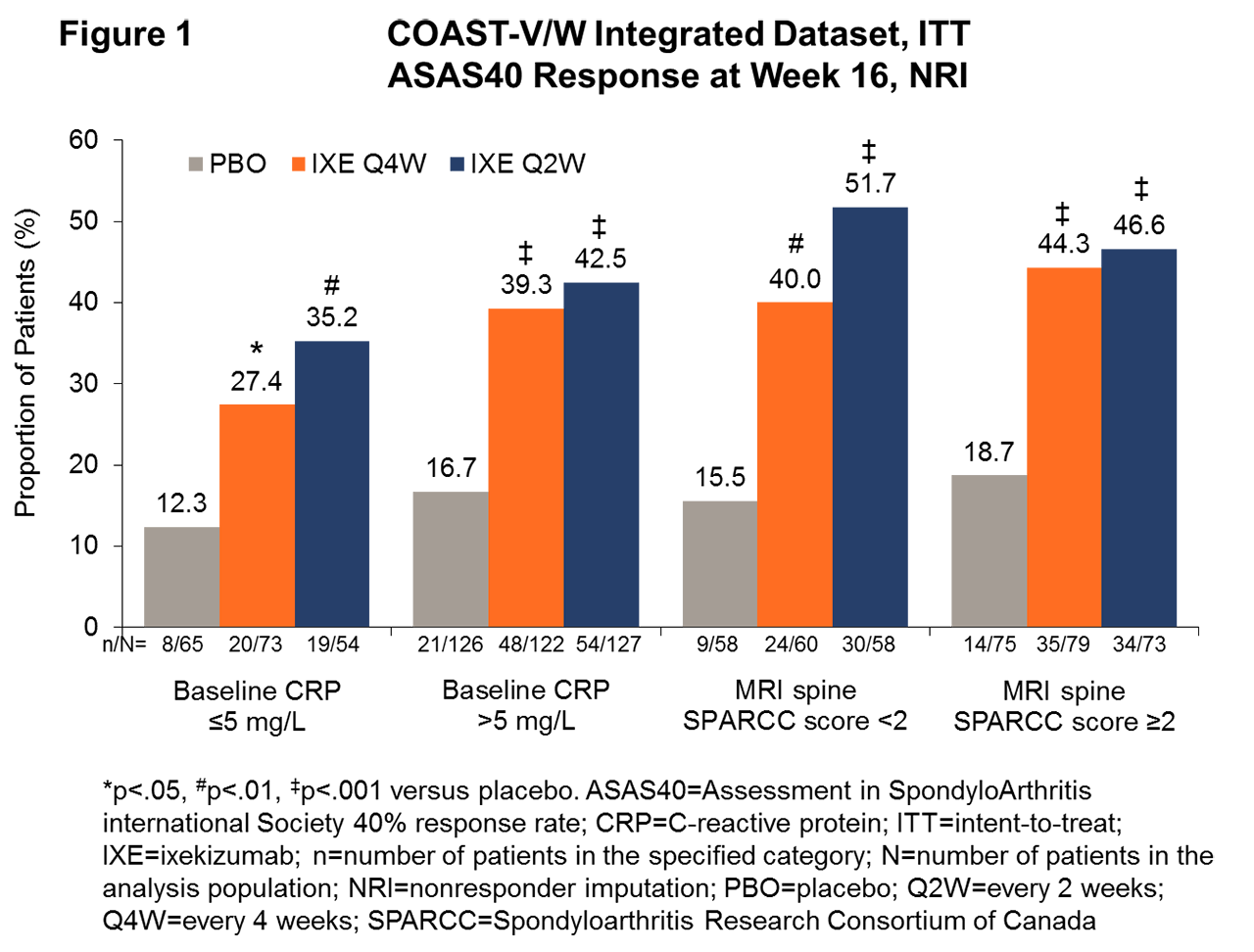Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: IL-17 plays an important role in the pathogenesis of radiographic axial spondyloarthritis (r-axSpA). Elevated CRP levels in serum predict response to TNF inhibitors (TNFi).1-4 The role of baseline spine MRI as a predictor of response has not been investigated for IL-17 inhibitors. This study evaluates response rates at week (wk) 16 with ixekizumab (IXE), an IL-17A antagonist, in patients with ankylosing spondylitis (AS)/r-axSpA and elevated or normal/low inflammation as measured by CRP or spinal MRI.
Methods: Two Phase 3, randomized, double-blind, placebo (PBO)-controlled trials (COAST-V, NCT02696785; COAST-W, NCT02696798) enrolled biologic-naive or TNFi-experienced patients, respectively, with active disease (BASDI ≥4 and spinal pain ≥4 on a numeric rating scale) and an established diagnosis of r-axSpA and fulfilling Assessment of SpondyloArthritis international Society (ASAS) criteria (sacroiliitis on radiograph by modified New York [mNY] criteria and ≥1 spondyloarthritis feature). All patients fulfilling ASAS criteria also fulfilled mNY criteria for AS. Patients were treated with IXE (80 mg every 2 or 4 wks [Q2W, Q4W]) or PBO; adalimumab (40 mg Q2W) was an active reference arm in COAST-V. We examined ASAS 40% (ASAS40) response rates at wk 16 for the intent-to-treat population by baseline CRP (≤5 or >5 mg/L) and/or MRI spine inflammation (Spondyloarthritis Research Consortium of Canada [SPARCC] spine score < 2 or ≥2). Baseline spine MRI (scored by central readers) was available in 96% of patients in COAST-V and 51% in COAST-W. Higher scores reflect greater baseline disease activity. Missing data for ASAS40 were imputed by nonresponder imputation.
Results: In the COAST-V/W integrated dataset that combined biologic-naive and TNFi-experienced populations, significantly more patients treated with IXE achieved ASAS40 response at wk 16 than with PBO in the baseline CRP elevated ( >5 mg/L) group (39.3%, 42.5%, and 16.7% for IXE Q4W, IXE Q2W, and PBO, respectively; p< .001 for IXE Q4W, IXE Q2W vs PBO) and in the baseline CRP normal (≤5 mg/L) group (27.4%, 35.2%, and 12.3% for IXE Q4W, IXE Q2W, and PBO, respectively; p< .05 for IXE Q4W, p< .01 for IXE Q2W vs PBO, Fig 1), and the magnitude of response with IXE between elevated vs normal CRP groups was not statistically significant. Notably, a significantly higher proportion of patients achieved ASAS40 at wk 16 with IXE than with PBO, regardless of whether MRI spine SPARCC scores were < 2 (40%, 51.7%, and 15.5% for IXE Q4W, IXE Q2W, and PBO, respectively; p< .01 for IXE Q4W, p< .001 for IXE Q2W vs PBO) or ≥2 (44.3%, 46.6%, and 18.7% for IXE Q4W, IXE Q2W, and PBO, respectively; p< .001 for IXE Q4W, IXE Q2W vs PBO, Fig 1). Among the patients (n=79) with MRI spine SPARCC score < 2 and CRP ≤5 mg/L, the ASAS40 responses at wk 16 were 13%, 29%, and 48% for PBO, IXE Q4W, and IXE Q2W, respectively, with statistically significantly greater improvement for IXE Q2W vs PBO (p< .05).
Conclusion: IXE demonstrated rapid efficacy in the treatment of AS/r-axSpA at wk 16 irrespective of baseline serum CRP levels or spinal MRI score.
References:
- Inman et al. 2008
- Braun et al. 2016
- de Vries et al. 2009
- Vastesaeger et al. 2011
To cite this abstract in AMA style:
Maksymowych W, Gallo G, Bolce R, Zhao F, Geneus V, Østergaard M, Tada K, Deodhar A, Gensler L. Ixekizumab Is Effective in the Treatment of Radiographic Axial Spondyloarthritis Regardless of the Level of C-Reactive Protein or Magnetic Resonance Imaging Scores [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/ixekizumab-is-effective-in-the-treatment-of-radiographic-axial-spondyloarthritis-regardless-of-the-level-of-c-reactive-protein-or-magnetic-resonance-imaging-scores/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/ixekizumab-is-effective-in-the-treatment-of-radiographic-axial-spondyloarthritis-regardless-of-the-level-of-c-reactive-protein-or-magnetic-resonance-imaging-scores/