Session Information
Date: Sunday, November 12, 2023
Title: (0252–0282) Miscellaneous Rheumatic & Inflammatory Diseases Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Cystic fibrosis (CF) is characterized by mutations within the CFTR (cystic fibrosis transmembrane conductance regulator) gene that result in a defect of the chloride transporter protein in different organs, particularly in the lung. Musculoskeletal symptoms have been reported in up to 29% of CF patients1, most frequently as recurrent episodes of mono- or polyarthritis in joints of hands and feet. Potent CFTR modulator therapies targeting the F508del mutation in CF have become available (i.e., Trikafta© – consisting of a triple combination of elexacaftor, tezacaftor, and ivacaftor) that increase CFTR protein availability.
We seek to characterize musculoskeletal symptoms in a cohort of consecutive CF patients.
Methods: 25 CF consecutive outpatients were enrolled in this monocentric, prospective, and cross-sectional cohort study. Rheumatologic evaluation included clinical and laboratory parameters. Data were analyzed by covariance (ANCOVA) models, using the general linear model approach. Correlation analyses were performed calculating nonparametric Spearman correlation rank coefficients.
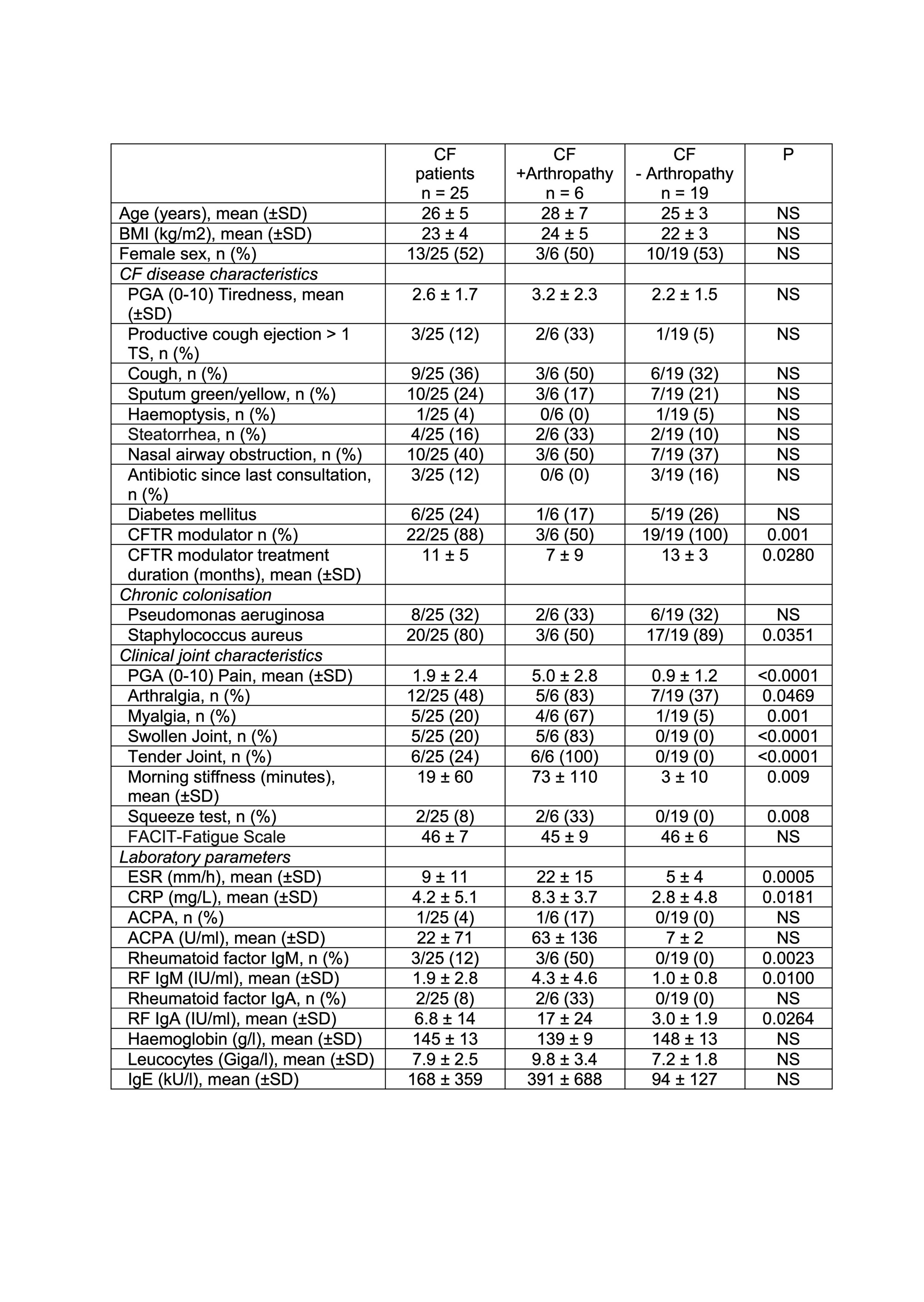
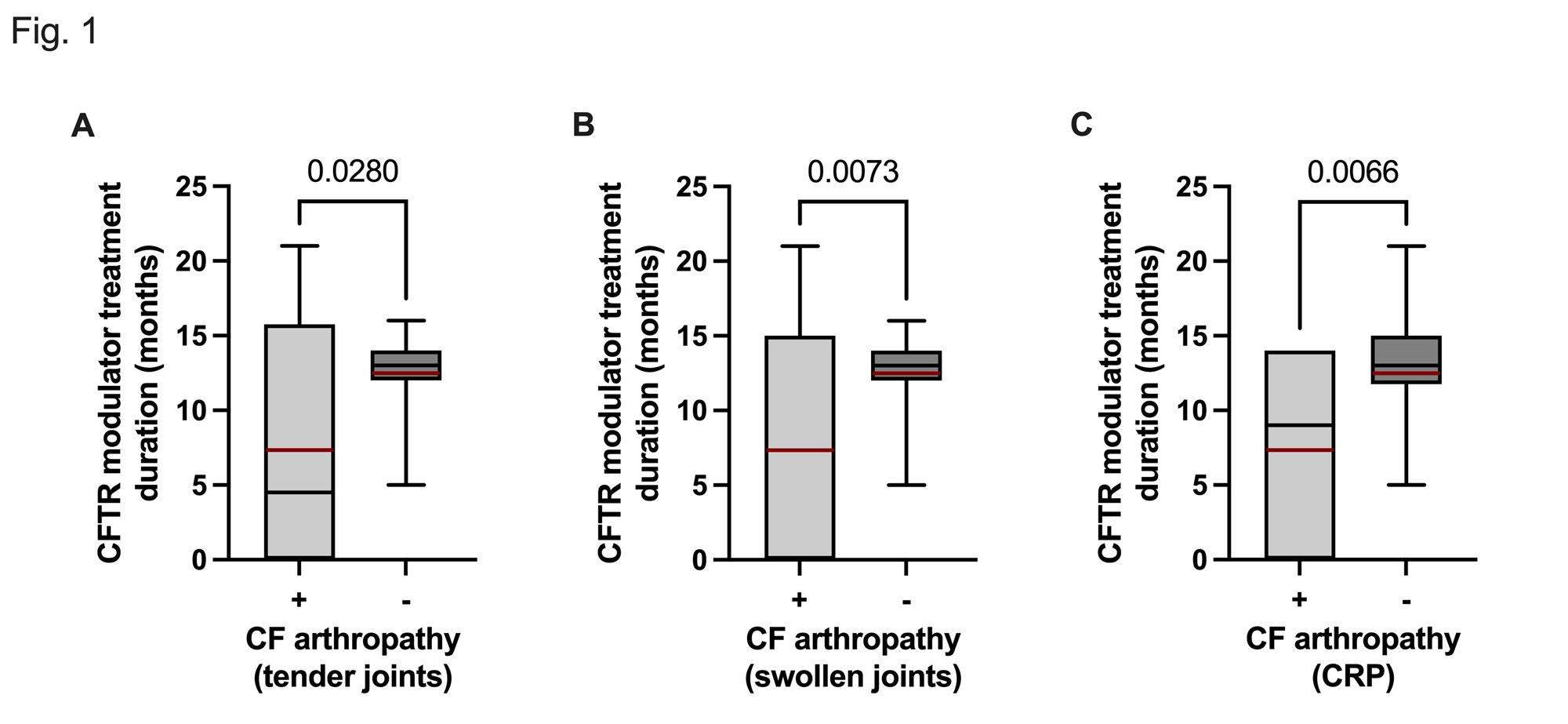
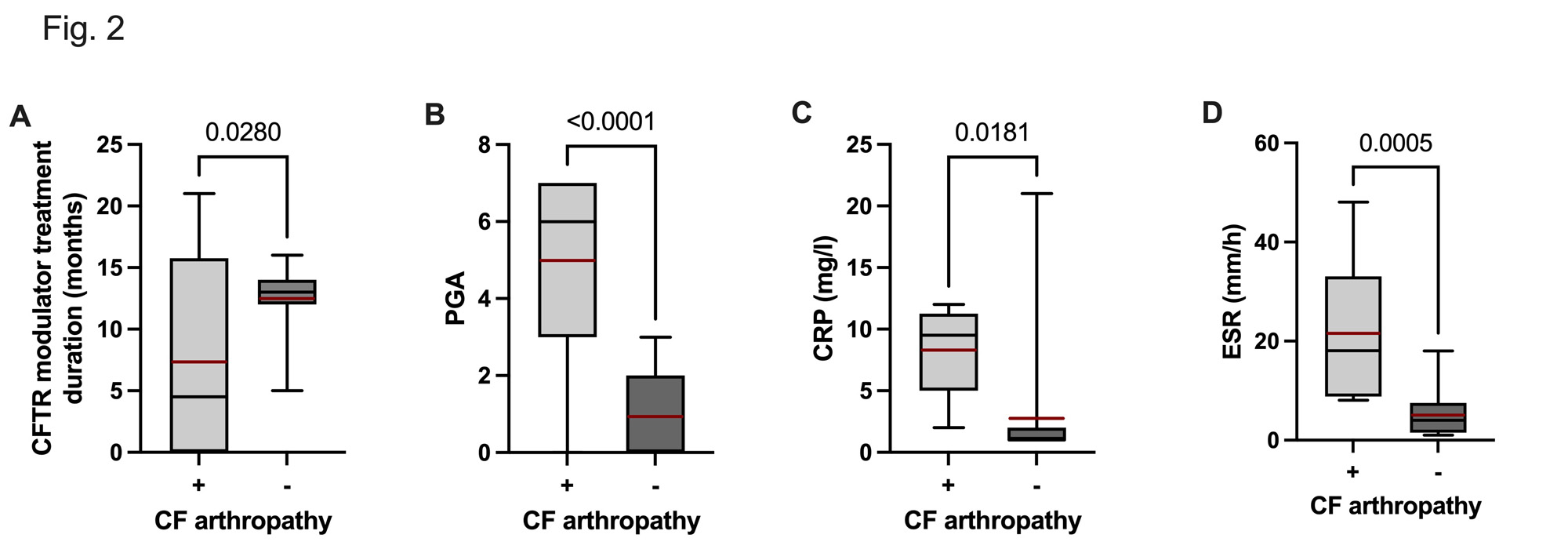
Results: Baseline characteristics are outlined in Table 1. 22/25 CF patients were under CFTR modulator treatment with a mean treatment duration of 11 ± 5 months. Arthralgias and myalgias were reported in 48% and 20% of patients, respectively. Arthritis, mainly involving small joints, was clinically detected in 6/25 (24%) patients and confirmed by ultrasound (US) in 3/6 patients. Self-reported myalgias, were significantly associated with the presence of swollen joints (r = 0.7452, p < 0.0001), tender joints, (r = 0.6674, p = 0.0003), a positive squeeze test (r = 0.5898, p = 0.0019) and morning stiffness (r = 0.6556, p = 0.0004) (Table 1). Disease activity as assessed by the SDAI was moderate (mean 18 + 3). CCP and rheumatoid factor (RF) were detected in one patient not on CFTR modulator therapy (with PIP synovitis confirmed by US). Two patients on CFTR modulator therapy tested positive for RF. Another patient was seronegative but synovitis was confirmed by US. Of note, longer duration of CFTR modulator therapy was significantly associated with a lower number of tender joints (r = -0.410 p = 0.054), swollen joints (r = -0.400, p = 0.048) and a lower CRP (r = -0.509, p = 0.048) (Fig 1 and Fig 2).
Conclusion: The current cohort study confirms that musculoskeletal symptoms are frequent in adult CF patients. Self-reported myalgias were significantly associated with arthritis mainly involving small joints. Interestingly, longer duration of CFTR modulator therapy was associated with a decreased number of tender and swollen joints in line with the assumption that amelioration of mucosal airway inflammation may decrease the risk of developing CF arthropathy.
References
Ja1. Janssen KMJ et al. Arthritis Res Ther 2015. doi: 10.1186/s13075-015-0690-6
To cite this abstract in AMA style:
Schmiedeberg K, Walter A, Joos L, Brutsche M, von Kempis J, Rubbert-Roth A. Is There a Decreased Risk of Developing CF Arthropathy in CF Patients Undergoing Treatment That Targets the F508del Mutation? [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/is-there-a-decreased-risk-of-developing-cf-arthropathy-in-cf-patients-undergoing-treatment-that-targets-the-f508del-mutation/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/is-there-a-decreased-risk-of-developing-cf-arthropathy-in-cf-patients-undergoing-treatment-that-targets-the-f508del-mutation/