Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: We reported that 7.7% (88/1137) of patients in an international inception cohort were ANA-negative at enrolment (Arthritis Care Res 2018 doi:1002/acr23712). There are no data on whether long-term outcomes or health care costs differ between ANA-negative versus ANA-positive patients. We compared damage accrual and costs between those who were ANA-negative versus ANA-positive at disease inception.
Methods: Patients fulfilling the revised ACR SLE Criteria from 33 centres in 11 countries were enrolled within 15 months of diagnosis. ANA at enrolment was detected by indirect immunofluorescence in a single laboratory; a positive ANA was defined as a titre ≥ 1/160. Data were collected annually on disease damage (SLICC/ACR Damage Index, SDI) and health care use (i.e., hospitalizations, medications, dialysis, and selected procedures) and supplemented by data on additional resource use and lost work-force/non-work-force productivity in a patient subset. Primary analyses included partial direct cost estimates based on the major health care components for the full sample. Estimates of complete direct and indirect costs were obtained by assuming that the ratios of total direct to partial direct costs and indirect to partial direct costs were the same for the full sample as for the subset. Health care use was costed using 2018 Canadian prices and lost productivity using Statistics Canada age-and-sex specific wages. The average annual rate of damage accrual and average annual costs over follow up were compared between patients who were ANA-negative versus ANA-positive at enrolment using multivariable regressions, adjusting for age and race/ethnicity.
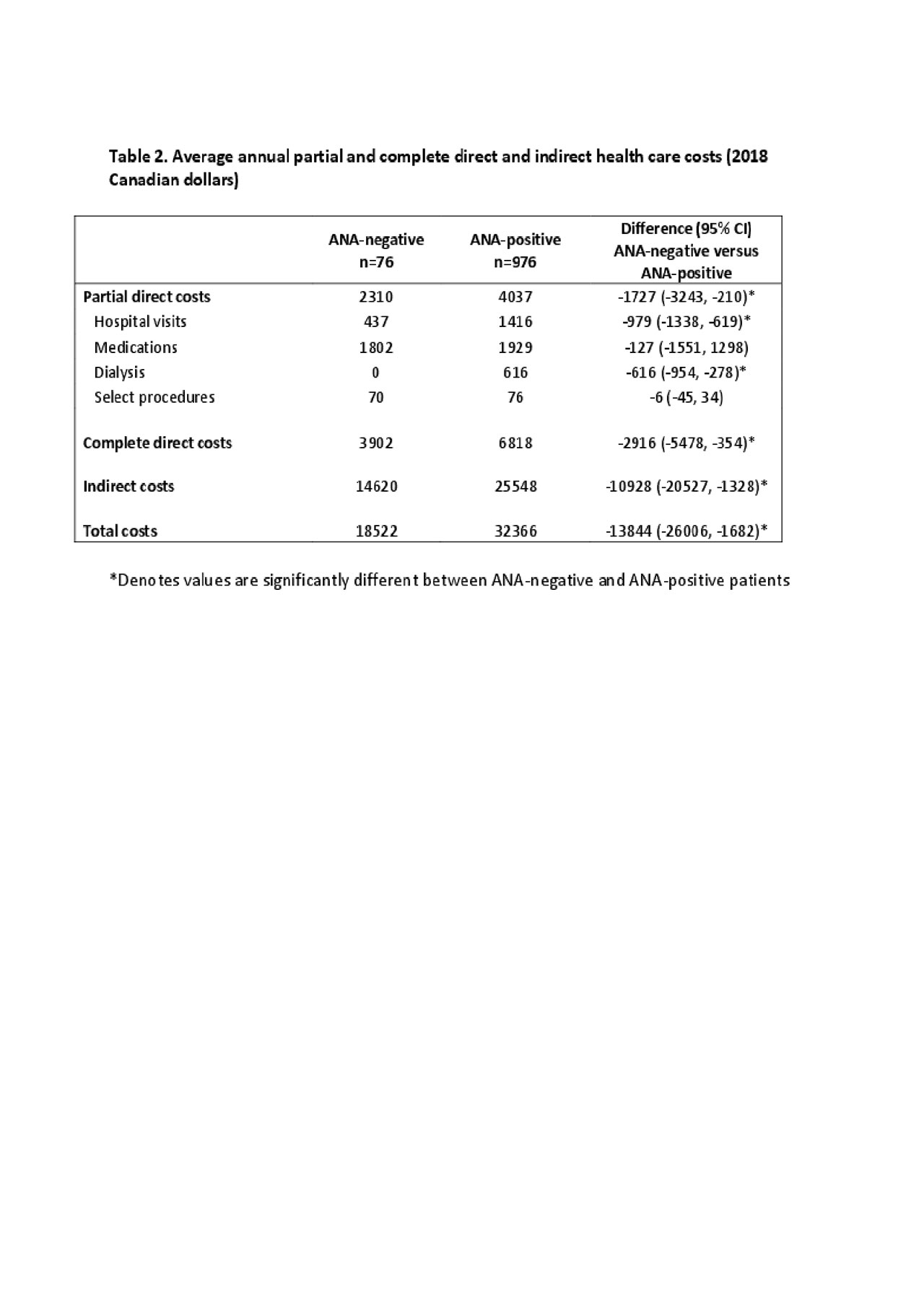
Results: 1052 of the 1137 patients provided cost data and were included; 76/1052 (7.2%) were ANA-negative at enrolment (Table 1). ANA-negative versus ANA-positive patients were older, more likely to be of Caucasian race/ethnicity, and less likely to be on immunosuppressants or have SLE-associated autoantibodies. Mean follow up of the ANA-negative versus ANA-positive was similar (8.6 years (range 0.8 – 17.8) versus 9.6 years (range 0.6 – 18.5). In the ANA-negative versus ANA-positive, the average annual rate of damage accrual was 0.10 versus 0.12 units/year; the difference was not significant in the univariable or multivariable analysis. In the ANA-negative versus ANA-positive, the average annual partial direct, complete direct, and indirect costs were significantly less ($2310 (95% CI 897, 3723), $3902 (95% CI 1516, 6287) and $14,620 (95% CI 5680, 23560) versus $4036 (95% CI 3428, 4645), $6818 (95% CI 5790, 7845) and $25,548 (95% CI 21698, 29398)) (Table 2). However, in multivariable analysis, wide CIs precluded definitive conclusions (β-coefficient for ANA-negativity, -$5684, 95% CI -$24258, $12890).
Conclusion: ANA-negative versus ANA-positive SLE patients at disease inception incurred lower costs in univariable analysis, but with adjustment for age and race/ethnicity, no differences could be detected. More research is required to characterize ANA-status over the disease and determine if costs/outcomes are more associated with persistent ANA-negativity/positivity, rather than ANA-status at disease inception.
To cite this abstract in AMA style:
Choi M, Barber M, Fritzler M, Hanly J, Urowitz M, St-Pierre Y, Romero-Diaz J, Gordon C, Bae S, Bernatsky S, Wallace D, Isenberg D, Rahman A, Ginzler E, Petri M, Bruce I, Fortin P, Gladman D, Sanchez-Guerrero J, Ramsey-Goldman R, Khamashta M, Aranow C, Mackay M, Alarcón G, Manzi S, Nived O, Jönsen A, Zoma A, van Vollenhoven R, Ramos-Casals M, Ruiz-Irastorza G, Lim S, Kalunian K, Inanc M, Kamen D, Peschken C, Jacobsen S, Askanase A, Farewell V, Clarke A. Is ANA-status at Disease Inception Associated with Long-term Damage Accrual and Direct and Indirect Health Care Costs in the Systemic Lupus International Collaborating Clinics (SLICC) Inception Cohort? [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/is-ana-status-at-disease-inception-associated-with-long-term-damage-accrual-and-direct-and-indirect-health-care-costs-in-the-systemic-lupus-international-collaborating-clinics-slicc-inception-cohort/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/is-ana-status-at-disease-inception-associated-with-long-term-damage-accrual-and-direct-and-indirect-health-care-costs-in-the-systemic-lupus-international-collaborating-clinics-slicc-inception-cohort/