Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: We lack accurate clinical tools to identify the degree of active renal inflammation in childhood-onset SLE (cSLE). In this study, we investigated urine S100A4 and podocyte proteins as biomarkers of lupus nephritis (LN) activity with two primary objectives, (1) assess ability of candidate urine biomarkers to reflect the NIH activity index (NIH-AI) on renal biopsy and (2) determine whether changes in candidate urine biomarkers correlate with renal clinical disease activity.
Methods: We selected LN patients from an ongoing cSLE registry at Cincinnati Children’s Hospital with urine available near time of biopsy and at either six and/or 12-months post-biopsy. Urine levels of S100A4, nephrin and synaptopodin were measured using commercial ELISAs. All patients met ACR classification criteria for SLE. Clinical and laboratory data were collected for patients at each time point. Spearman correlations were performed to assess the relationship between individual biomarkers and both NIH-AI and the renal domain of the SLEDAI (SLEDAI-R). We used the Mann-Whitney U test and Wilcoxon signed-rank test to determine differences in biomarker values at each time point.
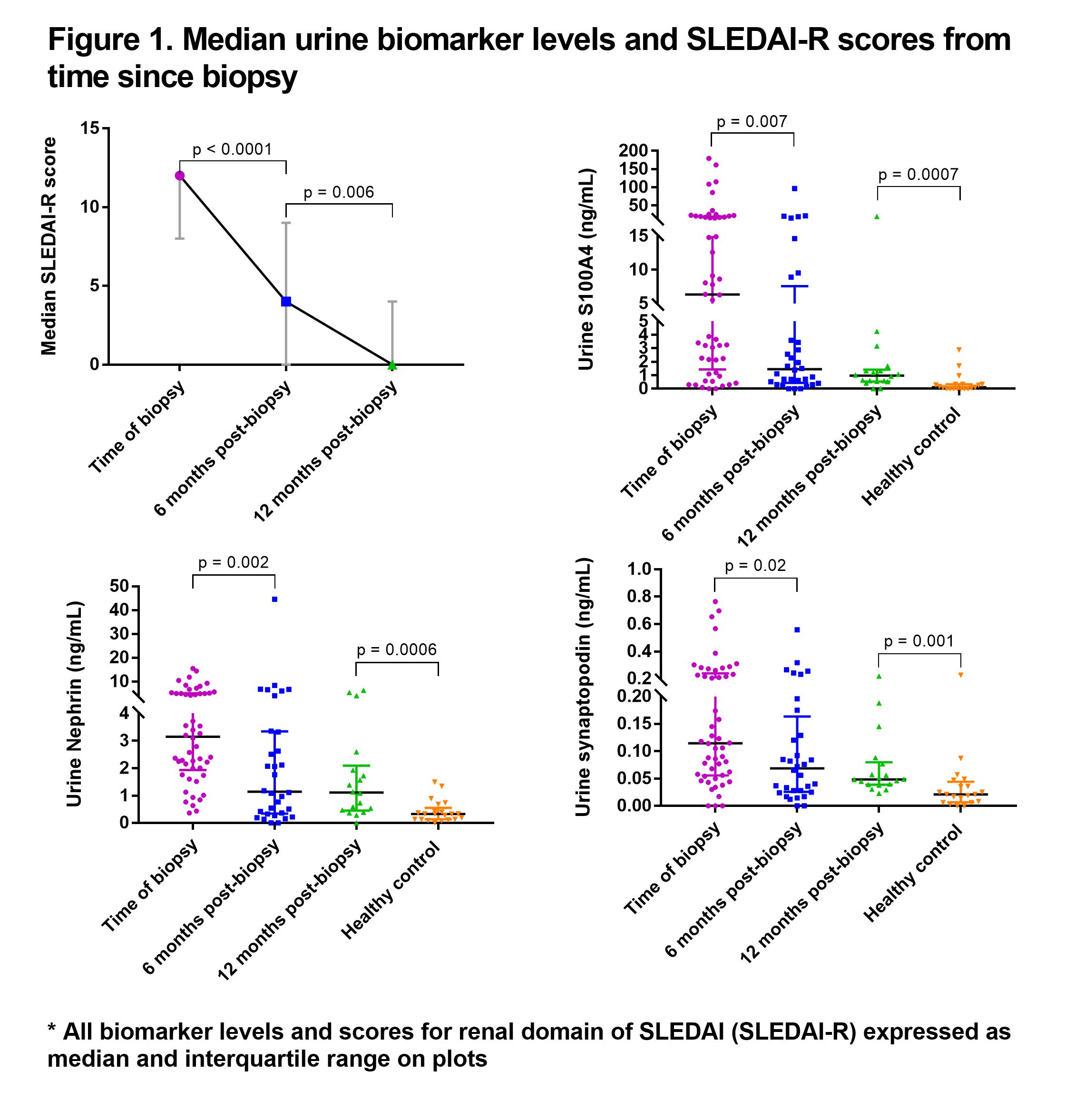
Results: There were 52, 32 and 18 patients with urine at renal biopsy, six-months and 12-months post-biopsy, respectively. Median urine S100A4, nephrin and synaptopodin levels in the entire cohort decreased from biopsy to six-months post-biopsy (Figure 1), but only urine S100A4 showed a significant decrease within individual patients between biopsy and six-months (median difference: -1.85 ng/mL, p = 0.01). There was no significant correlation between NIH-AI and any biomarker when evaluating the entire cohort together at biopsy. Upon stratifying patients by those with urine collected on biopsy day, synaptopodin showed a moderate correlation with NIH-AI (rs = 0.47, p = 0.027). Patients with class III/IV LN also had moderate correlation of nephrin with NIH-AI (rs = 0.37, p = 0.04). At six-months post-biopsy, there was a moderate correlation between all urine biomarkers and SLEDAI-R, rs = 0.48 (p = 0.007) for S100A4, rs = 0.53 (p = 0.003) for nephrin and rs = 0.47 (p = 0.01) for synaptopodin. Interestingly, the difference in S100A4 and SLEDAI-R levels between 12-months post-biopsy and biopsy showed moderate correlation (rs = 0.48, p = 0.045). Patients who had a complete clinical response by six or 12-months, as defined by a SLEDAI-R of zero, had lower S100A4 levels at follow-up than those with an incomplete clinical response (p = 0.0006).
Conclusion: While urine synaptopodin and nephrin levels appear to be better indicators of the NIH-AI on renal biopsy, S100A4 or change in S100A4 seems promising as a biomarker of individual clinical response to therapy post-biopsy. It remains to be determined if elevated levels of these biomarkers at follow-up indicate persistent histologic disease activity or poor histologic response to therapy.
To cite this abstract in AMA style:
Turnier J, Deng J, Avar Aydin PO, Witte D, Devarajan P, Fenchel M, Bennett M, Brunner HI. Investigating Urine S100A4 and Podocyte Proteins As Biomarkers of Lupus Nephritis Activity [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/investigating-urine-s100a4-and-podocyte-proteins-as-biomarkers-of-lupus-nephritis-activity/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/investigating-urine-s100a4-and-podocyte-proteins-as-biomarkers-of-lupus-nephritis-activity/