Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Systemic lupus erythematosus (SLE) is a heterogeneous autoimmune disease with unpredictable flares interspersed with prolonged periods of disease quiescence. Interferon (IFN) is a hallmark of disease and has been linked to disease flares, but the precise mechanism by which this occurs remain unclear. To address this question, we examined the association between IFN and the immune changes in flaring and quiescent SLE patients at a single cell level.
Methods: A 41-marker panel was developed that enabled measurement of IFN-induced proteins (IIPs) in peripheral blood immune populations using CyTOF.Fifteen healthy controls (HCs), 26 quiescent (clinical SLEDAI-2K = 0 for one year) and 42 recently flaring (< 1 month, change in clinical SLEDAI-2K ≥ 1 requiring escalation of therapy) SLE patients were examined. Expression of individual IIPs normalized to healthy controls, as well as a composite IIP score incorporating the expression of all seven proteins was assessed.
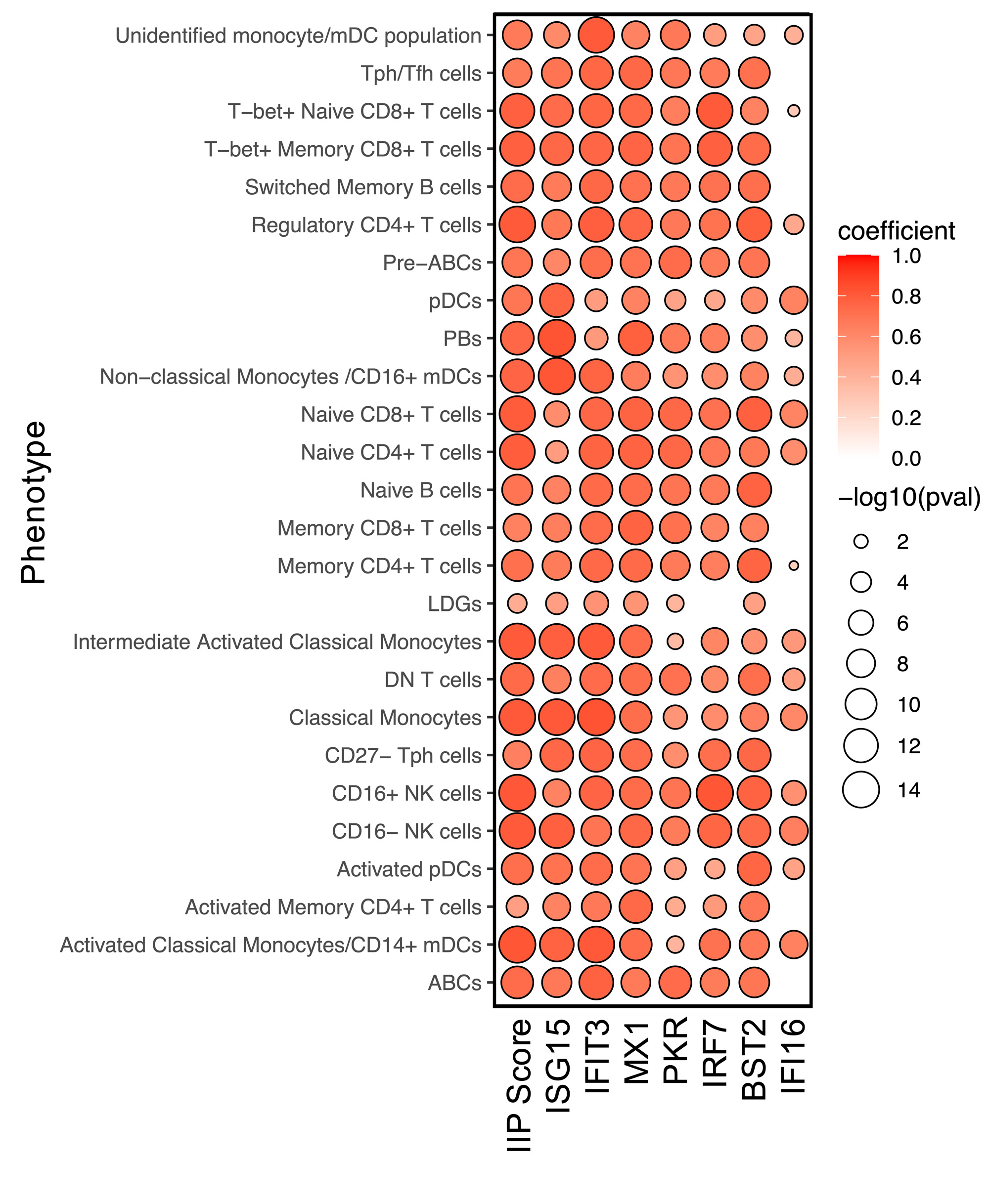
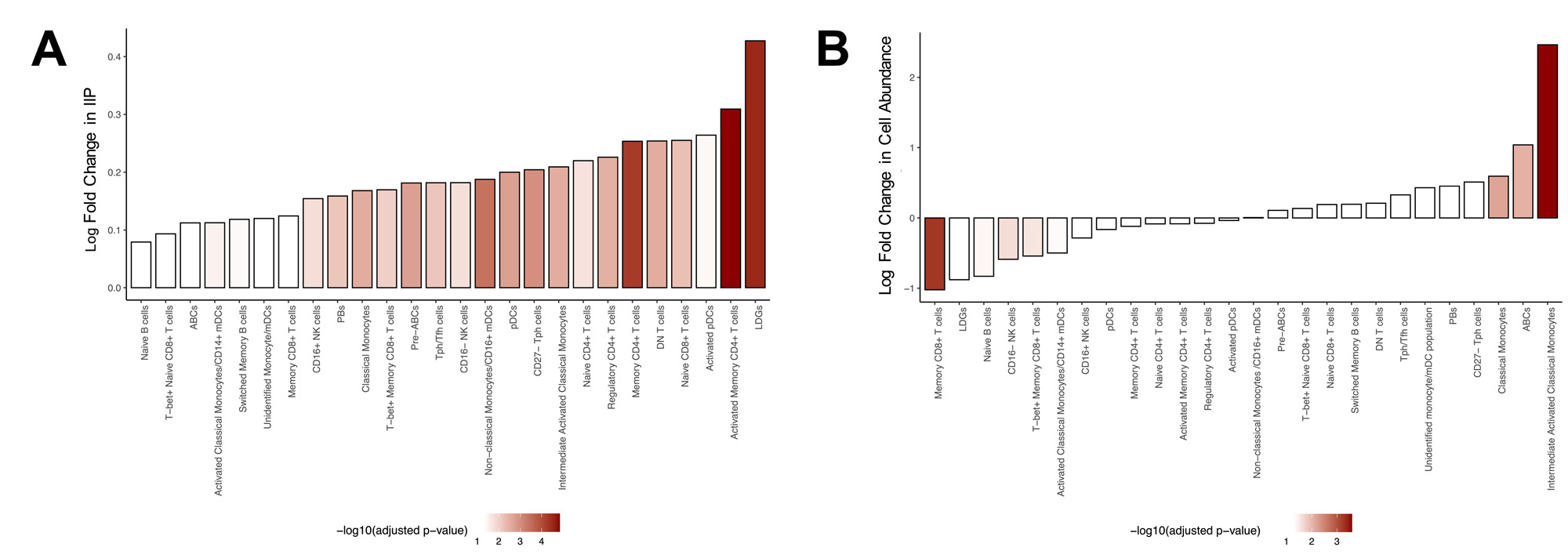
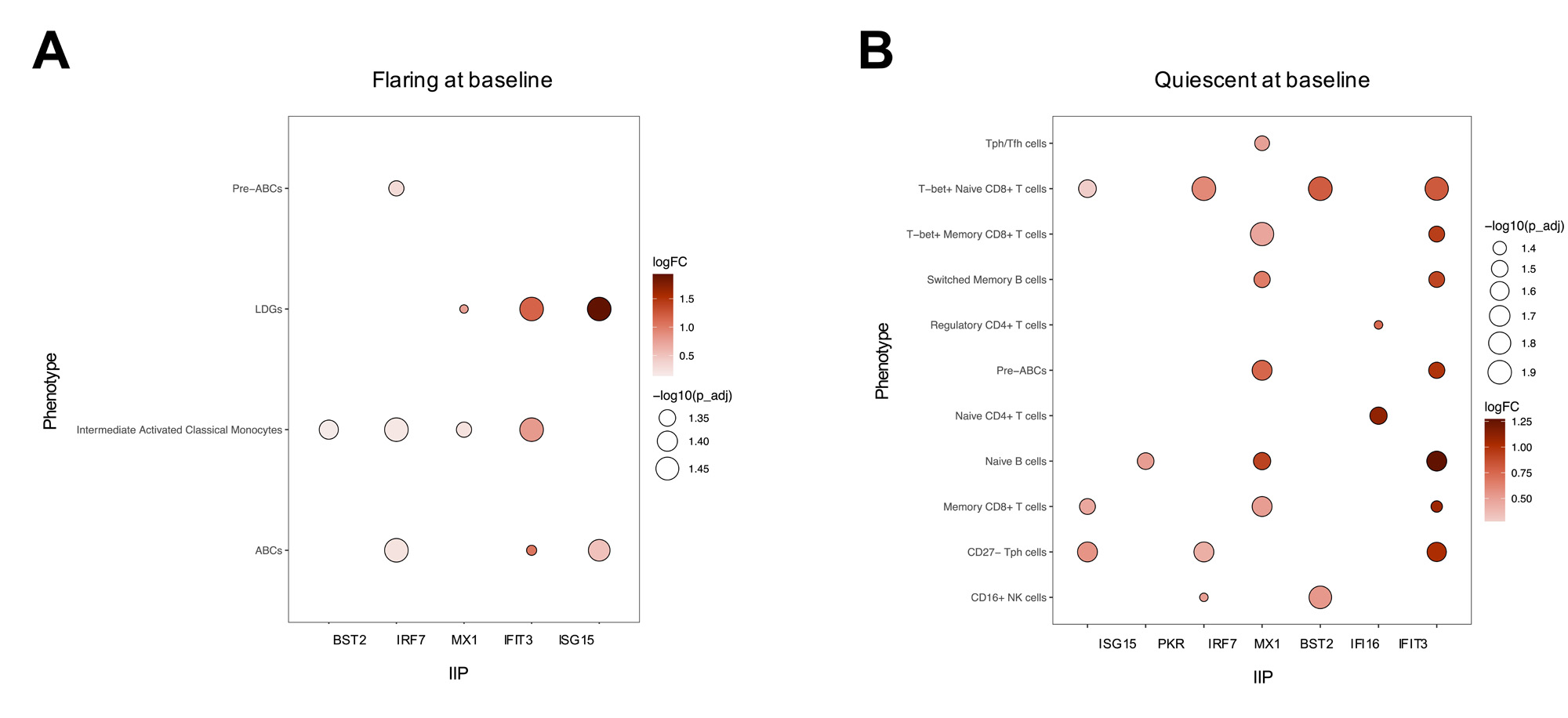
Results: Twenty-six distinct immune populations were identified, all of which demonstrated a strong correlation between IIP levels and the IIG score in SLE (Figure 1).In all immune populations, the levels of IIPs were significantly elevated in SLE patients compared to HCs. In most cell populations, the levels of IIPs were elevated in flaring compared to quiescent SLE patients (Figure 2A) and correlated with the clinical SLEDAI-2K. Comparison of the abundance of the 26 immune populations between flaring and quiescent patients revealed that only classical monocytes, intermediate activated classical monocytes, and age-associated B cells (ABCs) were significantly elevated in flaring patients (Figure 2B). Very limited changes were seen between these 2 groups of patients for markers of cellular activation, such as CD86, TLR7, TLR9, FOXP3, HLA-DR, Ki67, and PD1. In contrast, in many relevant immune populations, elevations of these markers were associated with IIP levels suggesting that IFN, rather than disease status (i.e., flare vs quiescence), is driving this activation. In patients who were flaring at baseline, elevations of IIPs in pre-ABCs, ABCs, low density granulocytes (LDGs), and intermediate activated monocytes were associated with clinical disease activity one year later (Figure 3A).In contrast, quiescent patients that subsequently flared and were active at one year had elevations of IIPs in multiple T and B cell populations (Figure 3B).
Conclusion: The findings suggest that IFN initiates flares by promoting B and T cell activation, while sustained or recurrent disease activity appears to be more dependent upon its impact on ABCs, LDGs, and monocytes.
To cite this abstract in AMA style:
Faheem Z, Boukhaled G, Manion K, Munoz-Grajales C, Nassar C, Kim M, Gladman D, Urowitz M, Touma Z, Brooks D, Wither J. Investigating the Role of Interferon in Promoting Flares of SLE at a Single Cell Level [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/investigating-the-role-of-interferon-in-promoting-flares-of-sle-at-a-single-cell-level/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/investigating-the-role-of-interferon-in-promoting-flares-of-sle-at-a-single-cell-level/