Session Information
Date: Sunday, November 13, 2022
Title: Vasculitis – ANCA-Associated Poster II: Treatment Efficacy, Clinical Outcomes, Biomarkers
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Anti neutrophil cytoplasmic antibodies (ANCA)-associated vasculitis (AAV) includes granulomatosis with polyangiitis (GPA), eosinophilic granulomatosis with polyangiitis (EGPA) or microscopic polyarteritis (MPA). Standard treatment is often accompained by significant adverse events. Intravenous immunoglobulins (IVIG) may constitute a therapeutic alternative, however, the data are scarce./
To assess the utility and safety of IVIG in AAV.
Methods: Observational study of patients with AAV from Spanish referral center treated with at least one cycle of IVIG since January of 2000 to December of 2021. AAV diagnosis was based on a compatible clinical presentation and: a) positive ANCA serology and/or b) histology. Disease activity was assessed with the Birmingham Vasculitis Activity Score (BVAS). The efficacy was studies by: a) clinical parameters, b) analytical features (CPR; ESR) and c) Glucocorticoids Sparring effect. Those variables were measured at one, six twelve and twenty-four months of IGIV treatment. The clinical improvement was on BVAS categories: Remission, Partial Response and Refractory. The administration schedule was 2g/Kg/IV on one to five days.
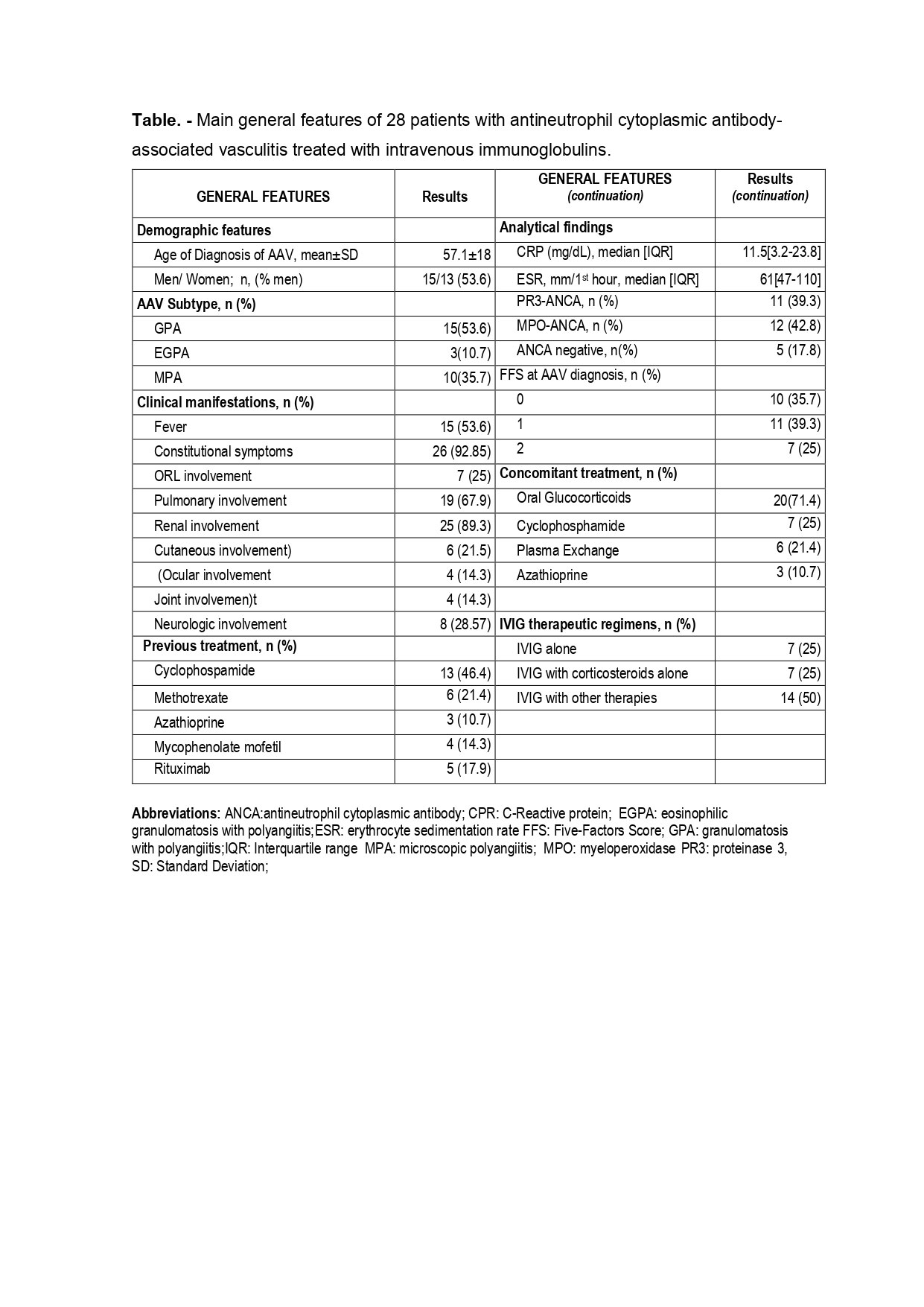
Results: We included a total of 28 patients; GPA (n=15), MPA (10), and EGPA (3). The main features are summarized in TABLE. The reasons for using IVIG were: a) relapse/refractory disease (n=25), b) presence/suspicion of infection (n=8).
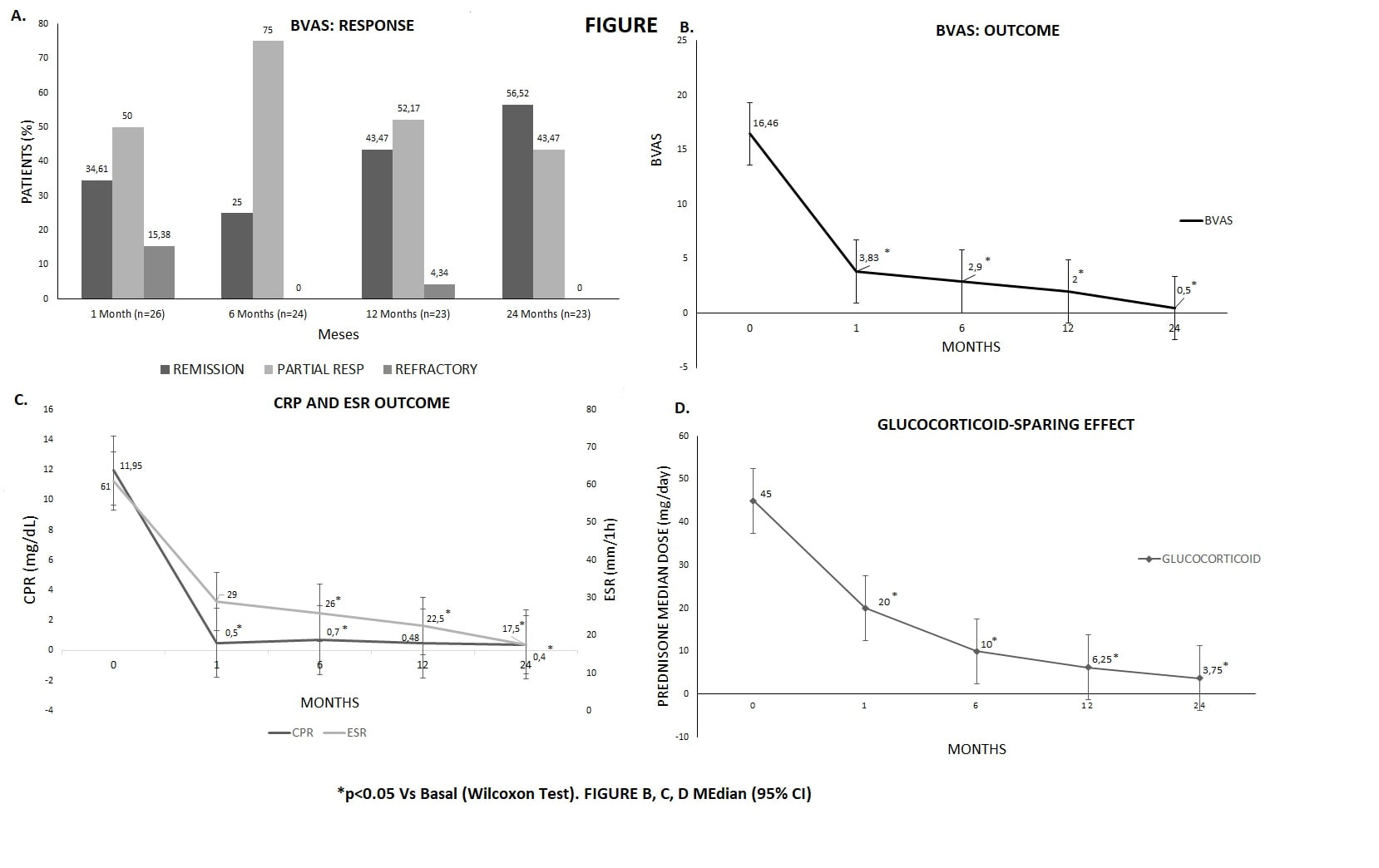
We observed a rapid and maintained Clinical improvement, since first month of IVIG onset, yielding a BVAS score of zero in 56.5% of patients at 24 months, also an imporvement on Glucocorticoids sparing effect (FIGURE) . The Adverse event were: headache (n=2), allergic reactions (n=2), Arterial Hypertension (n=1). Only in 1 patient developed congestive cardiac failure and had to stop the IVIG therapy. No Prothrombotic Events were observed. The administration schedule for the 2g/Kg/IV was: 1 g/Kg/IV in 2 days (n=4), 500 mg/Kg/IV in 4 days (n=16) and 400 mg/Kg/IV in 5 days (n=8).
Conclusion: IVIG seems to be an effectiveness and relative safe therapeutic option in relapse/refractory AAV or in presence of a concomitant infection.
To cite this abstract in AMA style:
Benavides Villanueva F, Corrales C, Calvo Río V, Loricera J, Aviles N, Blanco Madrigal J, Castañeda S, Gonzalez Gay M, Blanco R. Intravenous Immunoglobulin in Antineutrophil Cytoplasmic Antibody-associated Vasculitis: Study of 28 Cases from a Single Univeristary Hospital [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/intravenous-immunoglobulin-in-antineutrophil-cytoplasmic-antibody-associated-vasculitis-study-of-28-cases-from-a-single-univeristary-hospital/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/intravenous-immunoglobulin-in-antineutrophil-cytoplasmic-antibody-associated-vasculitis-study-of-28-cases-from-a-single-univeristary-hospital/