Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Pathological bone shape changes in the femur precede cartilage changes in knee OA and predict disease onset and progression to joint failure (Neogi 2013; Barr 2016). As reported previously, TPX-100-1, a randomized, double-blind, placebo-controlled, 12-month trial of IA TPX-100 in subjects with bilateral, mild-moderate (ICRS grades 2-3) patellofemoral OA, demonstrated statistically significant and clinically meaningful improvements in knee physical function (KOOS/WOMAC scales) in TPX-100-treated knees compared to placebo-exposed knees at 6 and 12 months (McGuire 2017). On quantitative MRI, only 14% of knees had measurable patella cartilage thickness changes, with no treatment differences demonstrated in this primary structural outcome measure. Post-hoc analyses revealed that 68 (73%) of these subjects also had moderate to severe (ICRS 2-4) bilateral tibiofemoral OA (TFOA), in whom similar significant clinical benefits in favor of TPX-100 were demonstrated (McGuire 2018). Study TPX-100-5 was designed to investigate MRI-based femoral bone shape changes in TPX-100-treated knees compared with placebo knees in subjects with bilateral moderate-severe TFOA at baseline.
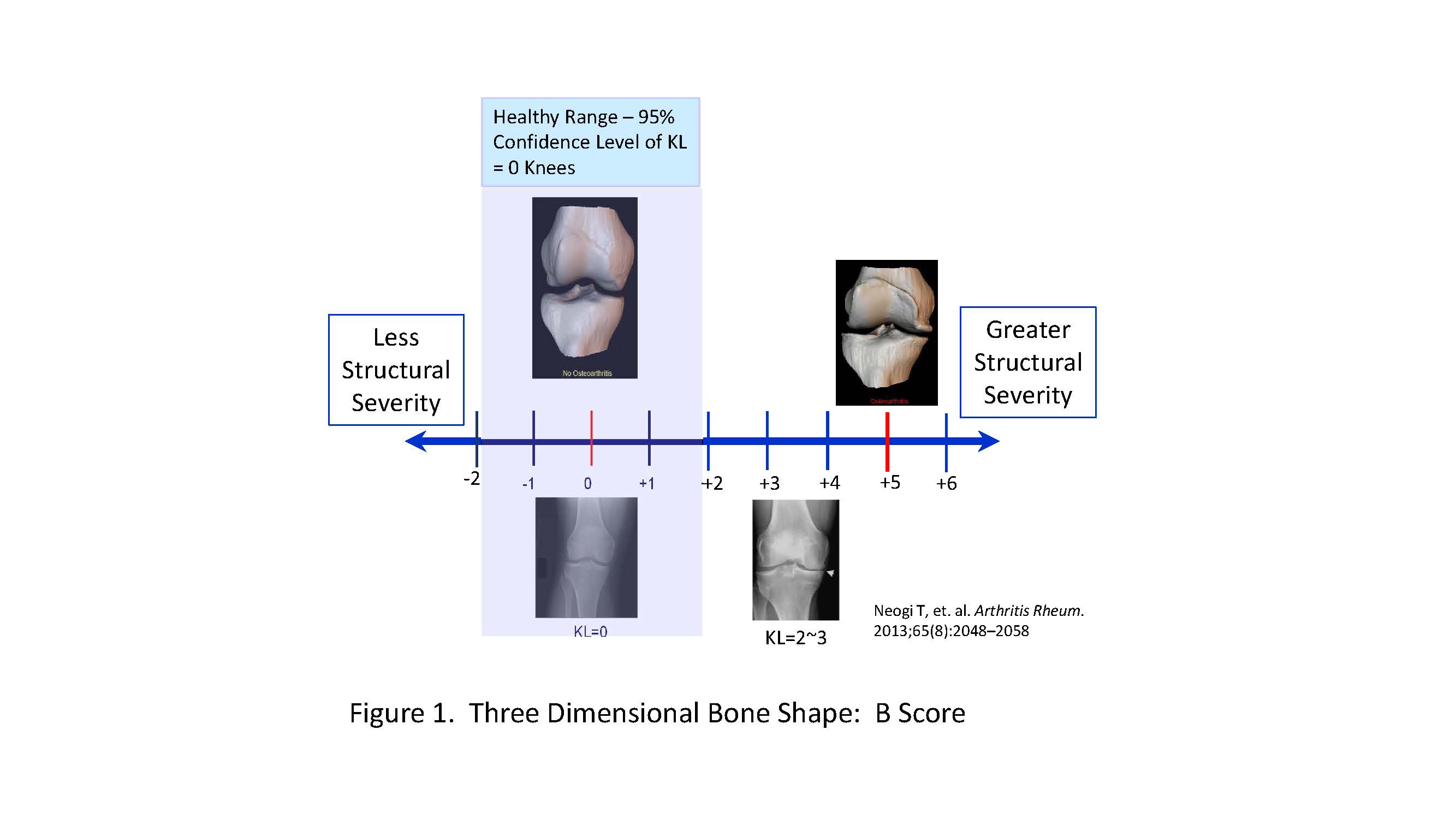
Methods: Bone shape changes from baseline to 6 and 12 months were analyzed by an automated method (Imorphics, Inc.) blind to treatment assignment, clinical and cartilage imaging results. Sufficient MRI image quality allowed analysis of bone shape changes in 55 of 68 subjects (81%) using active appearance model (AAM), a form of statistical shape analysis that can be used to discriminate OA versus non-OA shapes. Computed “B-Scores” range from -2 ( ‘non-OA’) to +6 (‘severe OA’) (Fig 1).
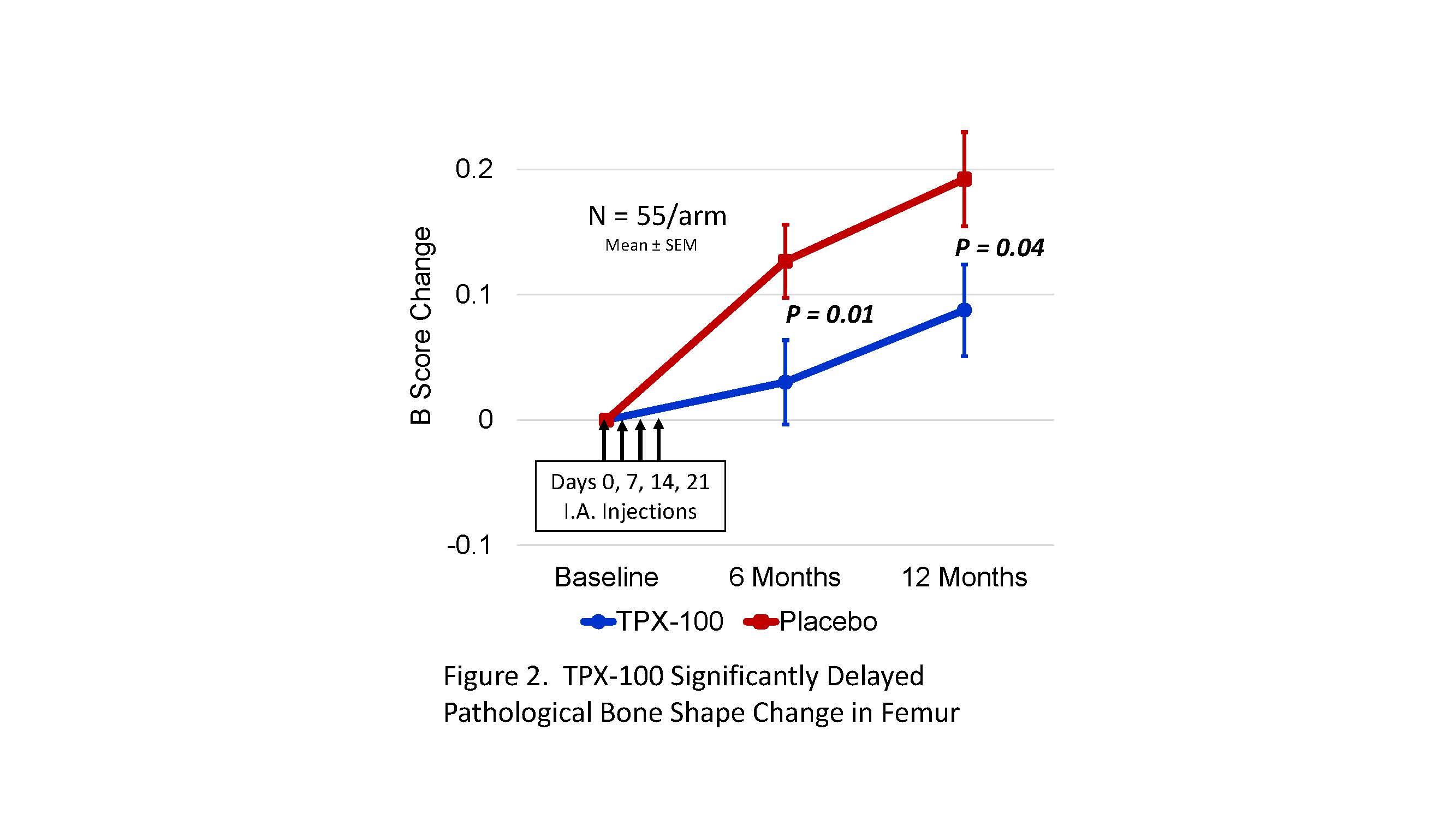
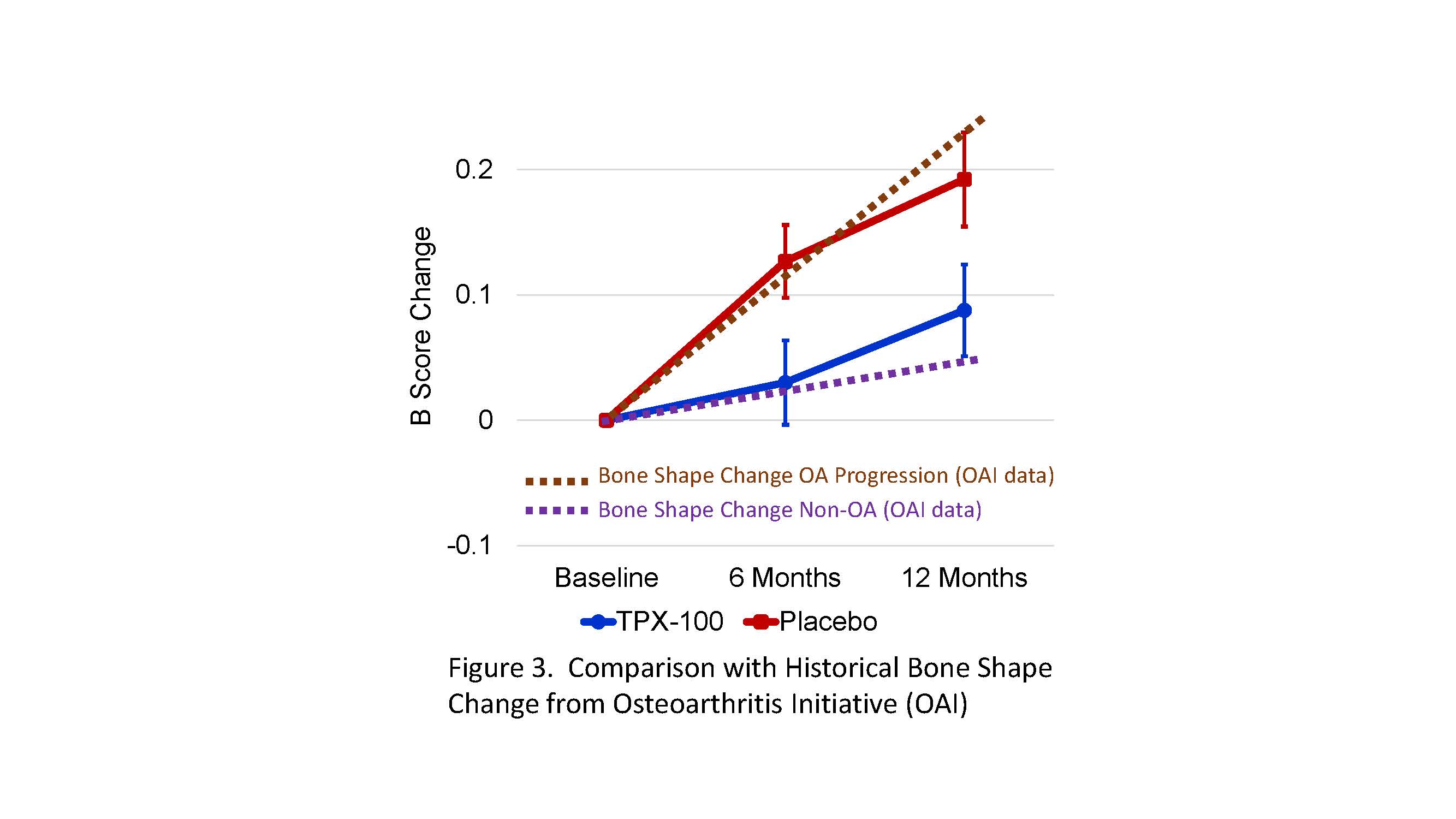
Results: MRIs of 110 knees from 55 subjects were analyzed. Of these, ≥91% had moderately-severe or severe (ICRS Grade 3 or 4) TFOA at baseline, equally distributed between TPX-100-treated and placebo-exposed knees. TPX-100-treated knees had significantly less bone shape change at 6 and 12 months (p=0.01 and P=0.04, respectively; Fig 2). Compared with historical controls from the OAI, the trajectory of bone-shape change in TPX-100-treated knees was similar to that observed in “non-OA” knees at 6 months, while that of placebo-exposed knees was similar to that of OA progressors (Fig 3).
Conclusion: Femoral bone shape change is progressive in normal aging and is accelerated in knee OA. Increasing change predicts worsening of symptomatic and radiographic knee OA and joint failure. After a single, 4-injection series (200 mg/injection), TPX-100 treatment in subjects with moderate to severe TFOA was associated with significantly slowed pathological femoral bone-shape change at 6 and 12 months compared with placebo. Delay or stabilization of structural pathology combined with robust and sustained improvement in physical knee function strongly supports further development of IA TPX-100 as a candidate DMOAD.
References
Neogi T, et. al. Arthritis Rheum. 2013;65(8):2048–2058
Barr AJ, et. al. Rheumatology 2016;55: 1585-1593
McGuire D. et. al. ACR/ARHP 2017 Abstract 13L
McGuire D. et. al. ACR/ARHP 2018 Abstract 5T113
McGuire_Femur Bone Shape_ Figure 1
McGuire_Femur Bone Shape_ Figure 2
McGuire_Femur Bone Shape_Figure 3
To cite this abstract in AMA style:
McGuire D, Bowes M, Brett A, Segal N, Miller M, Rosen D, Kumagai Y. Intra-Articular TPX-100 Significantly Delays Pathological Bone Shape Change at 6 and 12 Months in Moderate to Severe Tibiofemoral OA [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/intra-articular-tpx-100-significantly-delays-pathological-bone-shape-change-at-6-and-12-months-in-moderate-to-severe-tibiofemoral-oa/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/intra-articular-tpx-100-significantly-delays-pathological-bone-shape-change-at-6-and-12-months-in-moderate-to-severe-tibiofemoral-oa/
