Session Information
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Knee osteoarthritis (OA) is a common musculoskeletal disease associated with pain and functional impairment. There are few effective therapies, often limited by side-effects.1 This phase II study investigated the efficacy, safety, and pharmacokinetics of canakinumab, an anti-interleukin-1β antibody, in patients (pts) with knee OA (NCT01160822).
Methods: The study had a single placebo (PBO)-controlled ascending dose period assessing the safety of intra-articular (i.a.) canakinumab, and an 18-week randomized, double-blind, double-dummy PBO and naproxen-controlled period. Pts (N = 136) with symptomatic knee OA (VAS pain ≥40, Kellgren/Lawrence [K/L] grade 2 or 3) were enrolled to receive a single i.a. injection of canakinumab 600 mg or matched PBO at baseline (BL), and naproxen 500 mg twice daily or matched PBO. Co-primary endpoints were change from BL in knee OA pain (VAS 0-100] at Day 4 and in Western Ontario and McMaster OA Index (WOMAC) pain (scale 0-20) at Day 29.
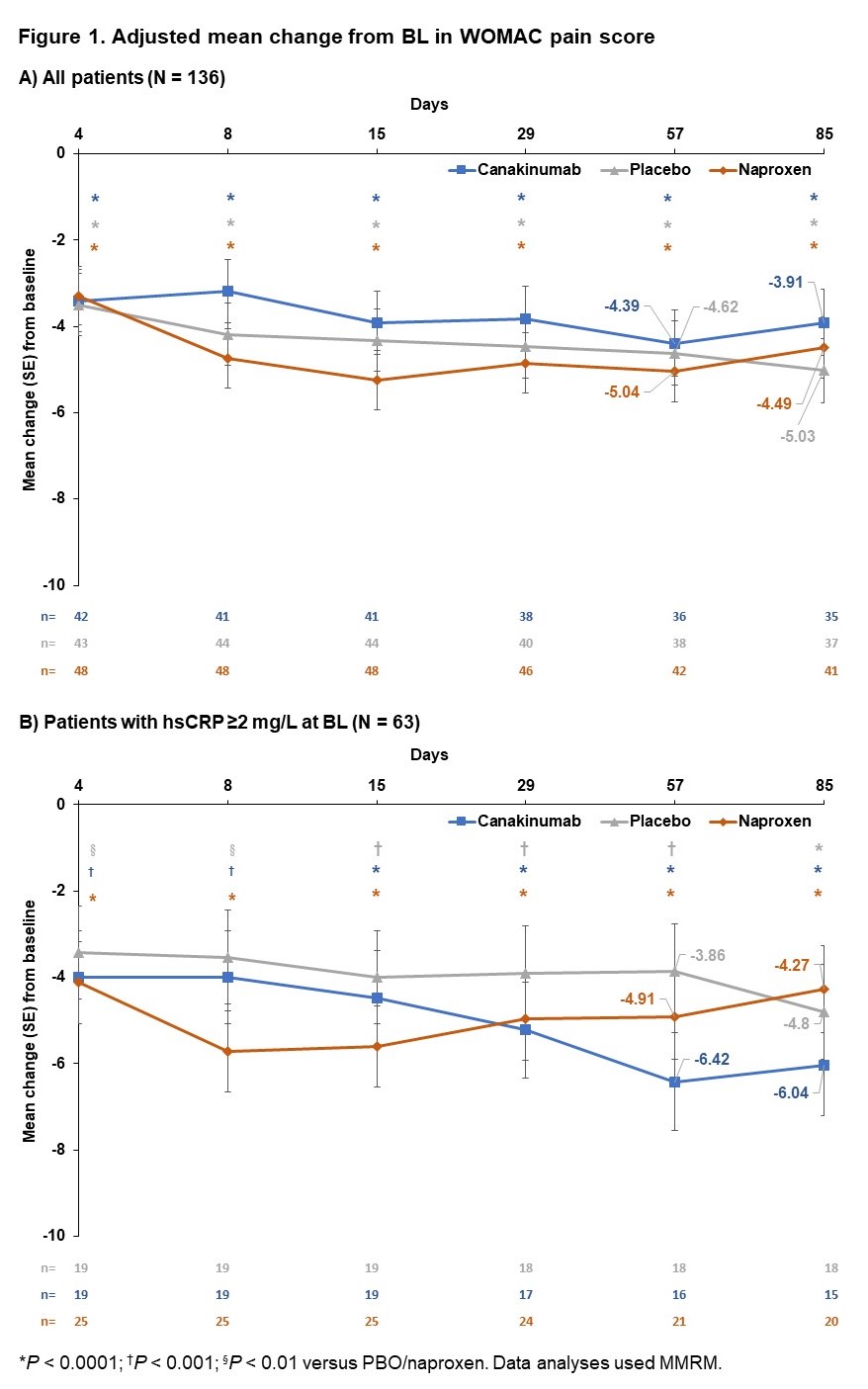
Results: Canakinumab i.a was generally safe and well tolerated and had a short resident time in the joint with an early detection (1 hour) in the circulation after i.a. injection. The co-primary efficacy endpoints were not met. The rapid and clinically significant decreases from BL in VAS pain and WOMAC pain scores were largely comparable between canakinumab, PBO and naproxen (Figure 1A). In a post hoc analysis of pts with BL elevated high sensitive C-reactive protein (hsCRP) levels (≥2 mg/L), canakinumab had a more pronounced inhibitory effect on pain than PBO or naproxen (-2.56 [-5.16; 0.04], P = 0.11; adjusted mean (90% CI) from a mixed-effect model repeated measures [MMRM]) at Day 57. Furthermore, this subpopulation showed a (-1.24 [-3.87; 1.39], P = 0.44) reduction of WOMAC pain at Day 85 compared to PBO or naproxen (Figure 1B).
Conclusion: The effect of canakinumab on pain in a population with knee OA was not different from PBO or naproxen. An effect of canakinumab on pain was observed in a subpopulation with elevated BL hsCRP levels. In addition to the data published previously on the CANTOS study2, these findings underscore the need to identify specific populations of pts with knee OA, who may benefit from anti-inflammatory treatment.
References: 1. Eymard F, Chevalier X. Rev Prat. 2019;69:515-9. 2. Schieker et al, Ann Intern Med. 2020;173:509-15.
To cite this abstract in AMA style:
Conaghan P, Chevalier X, Mindeholm L, Schramm U, Praestgaard J, Seroutou A, Roubenoff R, Schieker M. Intra-articular Canakinumab (anti-interleukin-1β) for Treatment of Symptomatic Knee Osteoarthritis: A Randomized, Double-blind, Placebo and Naproxen-controlled Phase II Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/intra-articular-canakinumab-anti-interleukin-1%ce%b2-for-treatment-of-symptomatic-knee-osteoarthritis-a-randomized-double-blind-placebo-and-naproxen-controlled-phase-ii-study/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/intra-articular-canakinumab-anti-interleukin-1%ce%b2-for-treatment-of-symptomatic-knee-osteoarthritis-a-randomized-double-blind-placebo-and-naproxen-controlled-phase-ii-study/